SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey
- PMID: 33515512
- PMCID: PMC7906675
- DOI: 10.1016/S2214-109X(20)30544-1
SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey
Abstract
Background: The first national severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serosurvey in India, done in May-June, 2020, among adults aged 18 years or older from 21 states, found a SARS-CoV-2 IgG antibody seroprevalence of 0·73% (95% CI 0·34-1·13). We aimed to assess the more recent nationwide seroprevalence in the general population in India.
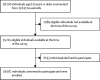
Methods: We did a second household serosurvey among individuals aged 10 years or older in the same 700 villages or wards within 70 districts in India that were included in the first serosurvey. Individuals aged younger than 10 years and households that did not respond at the time of survey were excluded. Participants were interviewed to collect information on sociodemographics, symptoms suggestive of COVID-19, exposure history to laboratory-confirmed COVID-19 cases, and history of COVID-19 illness. 3-5 mL of venous blood was collected from each participant and blood samples were tested using the Abbott SARS-CoV-2 IgG assay. Seroprevalence was estimated after applying the sampling weights and adjusting for clustering and assay characteristics. We randomly selected one adult serum sample from each household to compare the seroprevalence among adults between the two serosurveys.
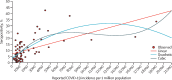
Findings: Between Aug 18 and Sept 20, 2020, we enrolled and collected serum samples from 29 082 individuals from 15 613 households. The weighted and adjusted seroprevalence of SARS-CoV-2 IgG antibodies in individuals aged 10 years or older was 6·6% (95% CI 5·8-7·4). Among 15 084 randomly selected adults (one per household), the weighted and adjusted seroprevalence was 7·1% (6·2-8·2). Seroprevalence was similar across age groups, sexes, and occupations. Seroprevalence was highest in urban slum areas followed by urban non-slum and rural areas. We estimated a cumulative 74·3 million infections in the country by Aug 18, 2020, with 26-32 infections for every reported COVID-19 case.
Interpretation: Approximately one in 15 individuals aged 10 years or older in India had SARS-CoV-2 infection by Aug 18, 2020. The adult seroprevalence increased approximately tenfold between May and August, 2020. Lower infection-to-case ratio in August than in May reflects a substantial increase in testing across the country.
Funding: Indian Council of Medical Research.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Government of India COVID-19 dashboard. Sept 30, 2020. https://www.mygov.in/covid-19
-
- WHO . World Health Organization; Geneva: 2020. A coordinated global research roadmap: 2019 novel coronavirus; March 2020.
-
- Wikipedia COVID-19 pandemic lockdown in India. Oct 2, 2020. https://en.wikipedia.org/wiki/COVID-19_pandemic_lockdown_in_India
-
- US Food & Drug Administration EUA authorized serology test performance. 2020. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

