The human-specific duplicated α7 gene inhibits the ancestral α7, negatively regulating nicotinic acetylcholine receptor-mediated transmitter release
- PMID: 33515545
- PMCID: PMC7949125
- DOI: 10.1016/j.jbc.2021.100341
The human-specific duplicated α7 gene inhibits the ancestral α7, negatively regulating nicotinic acetylcholine receptor-mediated transmitter release
Abstract
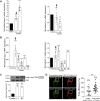
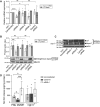
Gene duplication generates new functions and traits, enabling evolution. Human-specific duplicated genes in particular are primary sources of innovation during our evolution although they have very few known functions. Here we examine the brain function of one of these genes (CHRFAM7A) and its product (dupα7 subunit). This gene results from a partial duplication of the ancestral CHRNA7 gene encoding the α7 subunit that forms the homopentameric α7 nicotinic acetylcholine receptor (α7-nAChR). The functions of α7-nAChR in the brain are well defined, including the modulation of synaptic transmission and plasticity underlying normal attention, cognition, learning, and memory processes. However, the role of the dupα7 subunit remains unexplored at the neuronal level. Here, we characterize that role by combining immunoblotting, quantitative RT-PCR and FRET techniques with functional assays of α7-nAChR activity using human neuroblastoma SH-SY5Y cell variants with different dupα7 expression levels. Our findings reveal a physical interaction between dupα7 and α7 subunits in fluorescent protein-tagged dupα7/α7 transfected cells that negatively affects normal α7-nAChR activity. Specifically, in both single cells and cell populations, the [Ca2+]i signal and the exocytotic response induced by selective stimulation of α7-nAChR were either significantly inhibited by stable dupα7 overexpression or augmented after silencing dupα7 gene expression with specific siRNAs. These findings identify a new role for the dupα7 subunit as a negative regulator of α7-nAChR-mediated control of exocytotic neurotransmitter release. If this effect is excessive, it would result in an impaired synaptic transmission that could underlie the neurocognitive and neuropsychiatric disorders associated with α7-nAChR dysfunction.
Keywords: CHRFAM7A; calcium intracellular release; central nervous system; dupα7 nicotinic subunit; human genetics; human-specific duplicate genes; neurotransmitter release; α7 nicotinic acetylcholine receptors.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Fasoli F., Gotti C. Structure of neuronal nicotinic receptors. Curr. Top. Behav. Neurosci. 2015;23:1–17. - PubMed
-
- Bertrand D., Lee C.L., Flood D., Marger F., Donnelly-Roberts D. Therapeutic potential of α7 nicotinic acetylcholine receptors. Pharmacol. Rev. 2015;67:1025–1073. - PubMed
-
- Vijayaraghavan S., Pugh P.C., Zhang Z.W., Rathouz M.M., Berg D.K. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

