Membrane therapy using DHA suppresses epidermal growth factor receptor signaling by disrupting nanocluster formation
- PMID: 33515553
- PMCID: PMC7933808
- DOI: 10.1016/j.jlr.2021.100026
Membrane therapy using DHA suppresses epidermal growth factor receptor signaling by disrupting nanocluster formation
Abstract
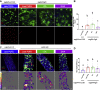
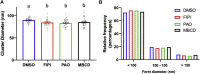
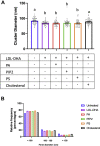
Epidermal growth factor receptor (EGFR) signaling drives the formation of many types of cancer, including colon cancer. Docosahexaenoic acid (DHA, 22∶6Δ4,7,10,13,16,19), a chemoprotective long-chain n-3 polyunsaturated fatty acid suppresses EGFR signaling. However, the mechanism underlying this phenotype remains unclear. Therefore, we used super-resolution microscopy techniques to investigate the mechanistic link between EGFR function and DHA-induced alterations to plasma membrane nanodomains. Using isogenic in vitro (YAMC and IMCE mouse colonic cell lines) and in vivo (Drosophila, wild type and Fat-1 mice) models, cellular DHA enrichment via therapeutic nanoparticle delivery, endogenous synthesis, or dietary supplementation reduced EGFR-mediated cell proliferation and downstream Ras/ERK signaling. Phospholipid incorporation of DHA reduced membrane rigidity and the size of EGFR nanoclusters. Similarly, pharmacological reduction of plasma membrane phosphatidic acid (PA), phosphatidylinositol-4,5-bisphosphate (PIP2) or cholesterol was associated with a decrease in EGFR nanocluster size. Furthermore, in DHA-treated cells only the addition of cholesterol, unlike PA or PIP2, restored EGFR nanoscale clustering. These findings reveal that DHA reduces EGFR signaling in part by reshaping EGFR proteolipid nanodomains, supporting the feasibility of using membrane therapy, i.e., dietary/drug-related strategies to target plasma membrane organization, to reduce EGFR signaling and cancer risk.
Keywords: Cancer; Cholesterol; Membranes/Fluidity; Omega-3 fatty acids; Receptors/Plasma membrane; Super-resolution microscopy.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Markman B., Javier Ramos F., Capdevila J., Tabernero J. EGFR and KRAS in colorectal cancer. Adv. Clin. Chem. 2010;51:71–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

