Rapid detection of SARS-CoV-2 by pulse-controlled amplification (PCA)
- PMID: 33515664
- PMCID: PMC7839394
- DOI: 10.1016/j.jviromet.2021.114083
Rapid detection of SARS-CoV-2 by pulse-controlled amplification (PCA)
Abstract
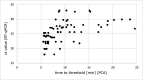
In the current pandemic of SARS-CoV-2, rapid identification of infected individuals is crucial for management and control of the outbreak. However, transport of samples, sample processing and RT-qPCR analysis in laboratories are time-consuming. Here we present a prototype of a novel nucleic acid-based test format - pulse controlled amplification - that allows detection of SARS-CoV-2 directly from up to eight swab samples simultaneously without the need for RNA extraction within 25 min with a sensitivity of 100 % for samples with a viral load of ≥ 1.6 × 10e3 copies/μl This new principle might pave the way to rapid and sensitive point of care testing.
Keywords: COVID-19; Diagnostics; Point of care; Rapid test; SARS-CoV-2.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Basu A., Zinger T., Inglima K., Woo K., Atie O., Yurasits L., See B., Aguero-Rosenfeld M.E. Performance of Abbott ID NOW COVID-19 rapid nucleic acid amplification test in nasopharyngeal swabs transported in viral media and dry nasal swabs, in a New York City academic institution (preprint) Microbiology. 2020 doi: 10.1101/2020.05.11.089896. - DOI - PMC - PubMed
-
- Behrmann O., Bachmann I., Spiegel M., Schramm M., El Wahed A.A., Dobler G., Dame G., Hufert F.T. Rapid detection of SARS-CoV-2 by low volume real-time single tube reverse transcription recombinase polymerase amplification using an exo probe with an internally linked quencher (exo-IQ) Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa116. hvaa116. - DOI - PMC - PubMed
-
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0513-4. - DOI - PMC - PubMed
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. - DOI - PMC - PubMed
-
- Dao Thi V.L., Herbst K., Boerner K., Meurer M., Kremer L.P.M., Kirrmaier D., Freistaedter A., Papagiannidis D., Galmozzi C., Klein S., Chandla P., Khalid D., Barreto Miranda I., Schnitzler P., Kraeusslich H.-G., Knop M., Anders S. Infectious Diseases (except HIV/AIDS) 2020. Screening for SARS-CoV-2 infections with colorimetric RT-LAMP and LAMP sequencing (preprint) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

