Loss of Mucosal p32/gC1qR/HABP1 Triggers Energy Deficiency and Impairs Goblet Cell Differentiation in Ulcerative Colitis
- PMID: 33515804
- PMCID: PMC8135049
- DOI: 10.1016/j.jcmgh.2021.01.017
Loss of Mucosal p32/gC1qR/HABP1 Triggers Energy Deficiency and Impairs Goblet Cell Differentiation in Ulcerative Colitis
Abstract
Background & aims: Cell differentiation in the colonic crypt is driven by a metabolic switch from glycolysis to mitochondrial oxidation. Mitochondrial and goblet cell dysfunction have been attributed to the pathology of ulcerative colitis (UC). We hypothesized that p32/gC1qR/HABP1, which critically maintains oxidative phosphorylation, is involved in goblet cell differentiation and hence in the pathogenesis of UC.
Methods: Ex vivo, goblet cell differentiation in relation to p32 expression and mitochondrial function was studied in tissue biopsies from UC patients versus controls. Functional studies were performed in goblet cell-like HT29-MTX cells in vitro. Mitochondrial respiratory chain complex V-deficient, ATP8 mutant mice were utilized as a confirmatory model. Nutritional intervention studies were performed in C57BL/6 mice.
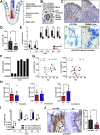
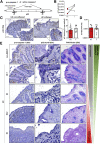
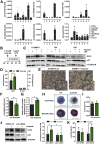
Results: In UC patients in remission, colonic goblet cell differentiation was significantly decreased compared to controls in a p32-dependent manner. Plasma/serum L-lactate and colonic pAMPK level were increased, pointing at high glycolytic activity and energy deficiency. Consistently, p32 silencing in mucus-secreting HT29-MTX cells abolished butyrate-induced differentiation and induced a shift towards glycolysis. In ATP8 mutant mice, colonic p32 expression correlated with loss of differentiated goblet cells, resulting in a thinner mucus layer. Conversely, feeding mice an isocaloric glucose-free, high-protein diet increased mucosal energy supply that promoted colonic p32 level, goblet cell differentiation and mucus production.
Conclusion: We here describe a new molecular mechanism linking mucosal energy deficiency in UC to impaired, p32-dependent goblet cell differentiation that may be therapeutically prevented by nutritional intervention.
Keywords: C1QBP; Inflammatory Bowel Disease; Mitochondrial Function; Mucus Barrier.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
Mitochondria in Ulcerative Colitis.Cell Mol Gastroenterol Hepatol. 2021;12(1):352-353. doi: 10.1016/j.jcmgh.2021.02.006. Epub 2021 Mar 6. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33684385 Free PMC article. No abstract available.
References
-
- Roediger W.E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712–715. - PubMed
-
- Sifroni K.G., Damiani C.R., Stoffel C., Cardoso M.R., Ferreira G.K., Jeremias I.C., Rezin G.T., Scaini G., Schuck P.F., Dal-Pizzol F., Streck E.L. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol Cell Biochem. 2010;342:111–115. - PubMed
-
- Santhanam S., Rajamanickam S., Motamarry A., Ramakrishna B.S., Amirtharaj J.G., Ramachandran A., Pulimood A., Venkatraman A. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2158–2168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

