Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm
- PMID: 33516155
- PMCID: PMC7846099
- DOI: 10.1210/endocr/bqaa171
Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm
Abstract
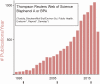
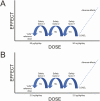
In 1997, the first in vivo bisphenol A (BPA) study by endocrinologists reported that feeding BPA to pregnant mice induced adverse reproductive effects in male offspring at the low dose of 2 µg/kg/day. Since then, thousands of studies have reported adverse effects in animals administered low doses of BPA. Despite more than 100 epidemiological studies suggesting associations between BPA and disease/dysfunction also reported in animal studies, regulatory agencies continue to assert that BPA exposures are safe. To address this disagreement, the CLARITY-BPA study was designed to evaluate traditional endpoints of toxicity and modern hypothesis-driven, disease-relevant outcomes in the same set of animals. A wide range of adverse effects was reported in both the toxicity and the mechanistic endpoints at the lowest dose tested (2.5 µg/kg/day), leading independent experts to call for the lowest observed adverse effect level (LOAEL) to be dropped 20 000-fold from the current outdated LOAEL of 50 000 µg/kg/day. Despite criticism by members of the Endocrine Society that the Food and Drug Administration (FDA)'s assumptions violate basic principles of endocrinology, the FDA rejected all low-dose data as not biologically plausible. Their decisions rely on 4 incorrect assumptions: dose responses must be monotonic, there exists a threshold below which there are no effects, both sexes must respond similarly, and only toxicological guideline studies are valid. This review details more than 20 years of BPA studies and addresses the divide that exists between regulatory approaches and endocrine science. Ultimately, CLARITY-BPA has shed light on why traditional methods of evaluating toxicity are insufficient to evaluate endocrine disrupting chemicals.
Keywords: biomonitoring; endocrine disruptor; guideline studies; low dose; nonmonotonic dose response; threshold.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Comment in
-
From Evidence of Harm to Public Health Policy: Is There Light at the End of the Tunnel? Response to: "Update on the Health Effects of bisphenol A: Overwhelming Evidence of Harm".Endocrinology. 2021 Mar 1;162(3):bqaa203. doi: 10.1210/endocr/bqaa203. Endocrinology. 2021. PMID: 33530103 Free PMC article. No abstract available.
References
-
- EPA. Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency, Chemical Assessment Summary, Bisphenol A; CASRN 80-05-7, 1988. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0356_summ.... Accessed July 9, 2020.
-
- FDA. Statement from Stephen Ostroff M.D., Deputy Commissioner for Foods and Veterinary Medicine, on National Toxicology Program draft report on Bisphenol A, February 23, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm598100.htm Accessed November 6, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

