Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease
- PMID: 33517890
- PMCID: PMC7849090
- DOI: 10.1186/s40168-020-00988-6
Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease
Erratum in
-
Correction: Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease.Microbiome. 2024 Jun 21;12(1):111. doi: 10.1186/s40168-024-01846-5. Microbiome. 2024. PMID: 38907261 Free PMC article. No abstract available.
Abstract
Background: Parkinson's disease (PD) is a neurodegenerative disorder with no absolute cure. The evidence of the involvement of gut microbiota in PD pathogenesis suggests the need to identify certain molecule(s) derived from the gut microbiota, which has the potential to manage PD. Osteocalcin (OCN), an osteoblast-secreted protein, has been shown to modulate brain function. Thus, it is of interest to investigate whether OCN could exert protective effect on PD and, if yes, whether the underlying mechanism lies in the subsequent changes in gut microbiota.
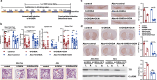
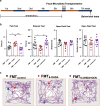
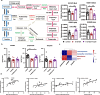
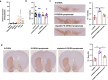
Results: The intraperitoneal injection of OCN can effectively ameliorate the motor deficits and dopaminergic neuronal loss in a 6-hydroxydopamine-induced PD mouse model. The further antibiotics treatment and fecal microbiota transplantation experiments confirmed that the gut microbiota was required for OCN-induced protection in PD mice. OCN elevated Bacteroidetes and depleted Firmicutes phyla in the gut microbiota of PD mice with elevated potential of microbial propionate production and was confirmed by fecal propionate levels. Two months of orally administered propionate successfully rescued motor deficits and dopaminergic neuronal loss in PD mice. Furthermore, AR420626, the agonist of FFAR3, which is the receptor of propionate, mimicked the neuroprotective effects of propionate and the ablation of enteric neurons blocked the prevention of dopaminergic neuronal loss by propionate in PD mice.
Conclusions: Together, our results demonstrate that OCN ameliorates motor deficits and dopaminergic neuronal loss in PD mice, modulating gut microbiome and increasing propionate level might be an underlying mechanism responsible for the neuroprotective effects of OCN on PD, and the FFAR3, expressed in enteric nervous system, might be the main action site of propionate. Video abstract.
Keywords: Gut microbiota; Osteocalcin; Parkinson’s disease; Propionate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

