An open clinical evaluation of selected siddha regimen in expediting the management of COVID-19 -a randomized controlled study
- PMID: 33519133
- PMCID: PMC7826002
- DOI: 10.1016/j.jaim.2021.01.002
An open clinical evaluation of selected siddha regimen in expediting the management of COVID-19 -a randomized controlled study
Abstract
Background: The corona virus disease 2019 (COVID-19), an acute respiratory disease, caused by a novel corona virus (SARS-CoV-2, previously known as 2019-nCoV), obtained worldwide attention. In this review, we explored the potential siddha strategies for COVID -19 infections.
Objectives: To evaluate the additional benefits of siddha drugs Vasantha kusumakaram mathirai, Thippili rasayanam, Adathodai manapagu and Kabasura kudineer compared to the allopathic standard treatment of care alone in COVID-19 asymptomatic, mild - moderate cases.
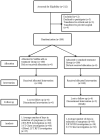
Materials and methods: The present study was an open label Two arm - randomized controlled interventional clinical study. The Group I patients were assigned to Siddha add on treatment whereas Group II subjects were assigned with standard treatment alone. The sample size was 100 for each group.
Result: The average number of days taken for reduction of symptoms showed significant results (P < 0.001) in Siddha add on compared with standard treatment. The real - time polymerase chain reaction (RT-PCR) investigation turned negative for 78.33% in Siddha add on and 33.33% in standard treatment after 11-14 days. Similarly, CT chest, covid pattern lung involvement percentage showed highly significant reduction (P < 0.0001) in Siddha add on treatment. In addition, Neutrophil Lymphocyte Ratio (NLR) ratio, showed significant reduction (P < 0.01) when analyzed by Wilcoxon signed rank test, and Renal, Liver parameters were within the normal limits in Siddha add on Group for 25 samples in post treatment.
Conclusion: Finally, it was concluded that Siddha add on Group showed accelerated recovery for COVID - 19 patients compared to standard Group. The synergistic effect of Siddha add on with standard treatment gave more promising results in the current study of COVID -19.
Keywords: Accelerated recovery; COVID-19; Siddha strategy; Synergistic effect.
© 2021 The Authors.
Conflict of interest statement
None
References
-
- World Health Organisation Corona virus disease Covid – 19. https://covid19.who.int/ Dashboard, updated on 22/8/2020.
-
- Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chan S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. https://doi:10.1186/s40779-020-00240-0 Published 2020 Mar 13. - DOI - PMC - PubMed
-
- The Siddha Formulary of India, Part I, Department of Health and Family Welfare, Government of India, 287-331.
-
- Kuppusamy Mudaliar K.N., Uththamarayan K.S. Vol. 40. Indian Medicine and Homeopathy Department; 2014. pp. 235–236. (Siddha VaithiyaThirattu).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous