Safety and Efficacy of Convalescent Plasma for Severe COVID-19: Interim Report of a Multicenter Phase II Study from Saudi Arabia
- PMID: 33519339
- PMCID: PMC7839574
- DOI: 10.4103/sjmms.sjmms_731_20
Safety and Efficacy of Convalescent Plasma for Severe COVID-19: Interim Report of a Multicenter Phase II Study from Saudi Arabia
Erratum in
-
Erratum: Safety and Efficacy of Convalescent Plasma for Severe COVID-19: Interim Report of a Multicenter Phase II Study from Saudi Arabia.Saudi J Med Med Sci. 2021 May-Aug;9(2):199. doi: 10.4103/1658-631X.315143. Saudi J Med Med Sci. 2021. PMID: 34084113 Free PMC article.
Abstract
Objective: To present the interim findings from a national study investigating the safety and efficacy of convalescent plasma (CP) containing detectable IgG antibodies as a treatment strategy for severe coronavirus disease 2019 (COVID-19).
Trial design and participants: An open label, two-arm, phase-II clinical trial conducted across 22 hospitals from Saudi Arabia. The intervention group included 40 adults (aged ≥18 years) with confirmed severe COVID-19 and the control group included 124 patients matched using propensity score for age, gender, intubation status, and history of diabetes and/or hypertension. Intervention group included those (a) with severe symptoms (dyspnea; respiratory rate, ≥30/min; SpO2, ≤93%, PaO2/FiO2 ratio, <300; and/or lung infiltrates >50% within 24-48 h), (b) requiring intensive care unit (ICU) care or (c) experiencing life-threatening conditions. The control group included confirmed severe COVID-19 patients of similar characteristics who did not consent for CP infusion or were not able to receive CP due to its nonavailability.
Interventions: The intervention group participants were infused 300 ml (200-400 ml/treatment dose) CP at least once, and if required, daily for up to 5 sessions, along with receiving the best standard of care. The control group only received the best standard of care.
Outcomes: The primary endpoints were safety and ICU length of stay (LOS). The secondary endpoints included 30-day mortality, days on mechanical ventilation and days to clinical recovery.
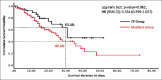
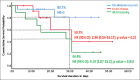
Results: CP transfusion did not result in any adverse effects. There was no difference in the ICU LOS (median 8 days in both groups). The mortality risk was lower in the CP group: 13% absolute risk reduction (P = 0.147), hazard ratio (95% confidence interval): 0.554 (0.299-1.027; P = 0.061) by log-rank test. There was no significant difference in the days on mechanical ventilation and days to clinical recovery.
Conclusion: CP containing detectable antibodies is a safe strategy and may result in a decrease in mortality in patients with severe COVID-19. The results of the completed trial with a larger study sample would provide more clarity if this difference in mortality is significant.
Trial registration: ClinicalTrials.gov Identifier: NCT04347681; Saudi Clinical Trials Registry No.: 20041102.
Keywords: Antibodies; COVID-19; SARS-CoV-2; Saudi Arabia; convalescent plasma.
Copyright: © 2020 Saudi Journal of Medicine & Medical Sciences.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous