Intrinsic Mechanisms Regulating Neuronal Migration in the Postnatal Brain
- PMID: 33519385
- PMCID: PMC7838331
- DOI: 10.3389/fncel.2020.620379
Intrinsic Mechanisms Regulating Neuronal Migration in the Postnatal Brain
Abstract
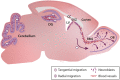
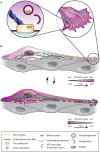
Neuronal migration is a fundamental brain development process that allows cells to move from their birthplaces to their sites of integration. Although neuronal migration largely ceases during embryonic and early postnatal development, neuroblasts continue to be produced and to migrate to a few regions of the adult brain such as the dentate gyrus and the subventricular zone (SVZ). In the SVZ, a large number of neuroblasts migrate into the olfactory bulb (OB) along the rostral migratory stream (RMS). Neuroblasts migrate in chains in a tightly organized micro-environment composed of astrocytes that ensheath the chains of neuroblasts and regulate their migration; the blood vessels that are used by neuroblasts as a physical scaffold and a source of molecular factors; and axons that modulate neuronal migration. In addition to diverse sets of extrinsic micro-environmental cues, long-distance neuronal migration involves a number of intrinsic mechanisms, including membrane and cytoskeleton remodeling, Ca2+ signaling, mitochondria dynamics, energy consumption, and autophagy. All these mechanisms are required to cope with the different micro-environment signals and maintain cellular homeostasis in order to sustain the proper dynamics of migrating neuroblasts and their faithful arrival in the target regions. Neuroblasts in the postnatal brain not only migrate into the OB but may also deviate from their normal path to migrate to a site of injury induced by a stroke or by certain neurodegenerative disorders. In this review, we will focus on the intrinsic mechanisms that regulate long-distance neuroblast migration in the adult brain and on how these pathways may be modulated to control the recruitment of neuroblasts to damaged/diseased brain areas.
Keywords: ATP/ADP; adult neurogenesis; autophagy; intrinsic mechanisms; neurodegenerative disorders; neuronal migration; olfactory bulb (OB).
Copyright © 2021 Bressan and Saghatelyan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

