Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum
- PMID: 33519389
- PMCID: PMC7843939
- DOI: 10.3389/fncir.2020.611841
Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum
Abstract
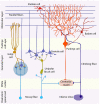
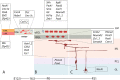
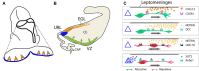
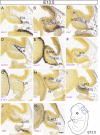
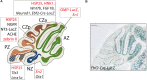
Granule cells (GCs) are the most numerous cell type in the cerebellum and indeed, in the brain: at least 99% of all cerebellar neurons are granule cells. In this review article, we first consider the formation of the upper rhombic lip, from which all granule cell precursors arise, and the way by which the upper rhombic lip generates the external granular layer, a secondary germinal epithelium that serves to amplify the upper rhombic lip precursors. Next, we review the mechanisms by which postmitotic granule cells are generated in the external granular layer and migrate radially to settle in the granular layer. In addition, we review the evidence that far from being a homogeneous population, granule cells come in multiple phenotypes with distinct topographical distributions and consider ways in which the heterogeneity of granule cells might arise during development.
Keywords: Bergmann glial fibers; cerebellum; compartmentation; external granular layer; granule cell; radial migration; upper rhombic lip.
Copyright © 2021 Consalez, Goldowitz, Casoni and Hawkes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
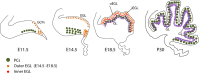

References
-
- Adams N. C., Tomoda T., Cooper M., Dietz G., Hatten M. E. (2002). Mice that lack astrotactin have slowed neuronal migration. Development 129, 965–972. - PubMed
-
- Aguilar A., Meunier A., Strehl L., Martinovic J., Bonniere M., Attie-Bitach T., et al. . (2012). Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/meckel syndrome. Proc. Natl. Acad. Sci. U S A 109, 16951–16956. 10.1073/pnas.1201408109 - DOI - PMC - PubMed
-
- Ahn A. H., Dziennis S., Hawkes R., Herrup K. (1994). The cloning of zebrin II reveals its identity with aldolase C. Development 120, 2081–2090. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

