AtxA-Controlled Small RNAs of Bacillus anthracis Virulence Plasmid pXO1 Regulate Gene Expression in trans
- PMID: 33519762
- PMCID: PMC7843513
- DOI: 10.3389/fmicb.2020.610036
AtxA-Controlled Small RNAs of Bacillus anthracis Virulence Plasmid pXO1 Regulate Gene Expression in trans
Abstract
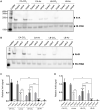
Small regulatory RNAs (sRNAs) are short transcripts that base-pair to mRNA targets or interact with regulatory proteins. sRNA function has been studied extensively in Gram-negative bacteria; comparatively less is known about sRNAs in Firmicutes. Here we investigate two sRNAs encoded by virulence plasmid pXO1 of Bacillus anthracis, the causative agent of anthrax. The sRNAs, named "XrrA and XrrB" (for pXO1-encoded regulatory RNA) are abundant and highly stable primary transcripts, whose expression is dependent upon AtxA, the master virulence regulator of B. anthracis. sRNA levels are highest during culture conditions that promote AtxA expression and activity, and sRNA levels are unaltered in Hfq RNA chaperone null-mutants. Comparison of the transcriptome of a virulent Ames-derived strain to the transcriptome of isogenic sRNA-null mutants revealed multiple 4.0- to >100-fold differences in gene expression. Most regulatory effects were associated with XrrA, although regulation of some transcripts suggests functional overlap between the XrrA and XrrB. Many sRNA-regulated targets were chromosome genes associated with branched-chain amino acid metabolism, proteolysis, and transmembrane transport. Finally, in a mouse model for systemic anthrax, the lungs and livers of animals infected with xrrA-null mutants had a small reduction in bacterial burden, suggesting a role for XrrA in B. anthracis pathogenesis.
Keywords: Bacillus; RNA-seq; anthracis; anthrax; gene expression; plasmid; sRNA; transcription.
Copyright © 2021 Corsi, Dutta, van Hoof and Koehler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Structural and Functional Analysis of Toxin and Small RNA Gene Promoter Regions in Bacillus anthracis.J Bacteriol. 2022 Sep 20;204(9):e0020022. doi: 10.1128/jb.00200-22. Epub 2022 Aug 31. J Bacteriol. 2022. PMID: 36043862 Free PMC article.
-
Direct Regulons of AtxA, the Master Virulence Regulator of Bacillus anthracis.mSystems. 2021 Aug 31;6(4):e0029121. doi: 10.1128/mSystems.00291-21. Epub 2021 Jul 20. mSystems. 2021. PMID: 34282944 Free PMC article.
-
In vivo characterization of an Hfq protein encoded by the Bacillus anthracis virulence plasmid pXO1.BMC Microbiol. 2017 Mar 14;17(1):63. doi: 10.1186/s12866-017-0973-y. BMC Microbiol. 2017. PMID: 28288571 Free PMC article.
-
Bacillus anthracis genetics and virulence gene regulation.Curr Top Microbiol Immunol. 2002;271:143-64. doi: 10.1007/978-3-662-05767-4_7. Curr Top Microbiol Immunol. 2002. PMID: 12224521 Review.
-
In vivo Bacillus anthracis gene expression requires PagR as an intermediate effector of the AtxA signalling cascade.Int J Med Microbiol. 2004 Apr;293(7-8):619-24. doi: 10.1078/1438-4221-00306. Int J Med Microbiol. 2004. PMID: 15149039 Review.
Cited by
-
Differential Chromosome- and Plasmid-Borne Resistance of Escherichia coli hfq Mutants to High Concentrations of Various Antibiotics.Int J Mol Sci. 2021 Aug 18;22(16):8886. doi: 10.3390/ijms22168886. Int J Mol Sci. 2021. PMID: 34445592 Free PMC article.
-
TaRTLEt: Transcriptionally-active Riboswitch Tracer Leveraging Edge deTection.PeerJ. 2025 May 26;13:e19418. doi: 10.7717/peerj.19418. eCollection 2025. PeerJ. 2025. PMID: 40444283 Free PMC article.
-
Structural and Functional Analysis of Toxin and Small RNA Gene Promoter Regions in Bacillus anthracis.J Bacteriol. 2022 Sep 20;204(9):e0020022. doi: 10.1128/jb.00200-22. Epub 2022 Aug 31. J Bacteriol. 2022. PMID: 36043862 Free PMC article.
-
PRD-Containing Virulence Regulators (PCVRs) in Pathogenic Bacteria.Front Cell Infect Microbiol. 2021 Oct 19;11:772874. doi: 10.3389/fcimb.2021.772874. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34737980 Free PMC article. Review.
-
Direct Regulons of AtxA, the Master Virulence Regulator of Bacillus anthracis.mSystems. 2021 Aug 31;6(4):e0029121. doi: 10.1128/mSystems.00291-21. Epub 2021 Jul 20. mSystems. 2021. PMID: 34282944 Free PMC article.
References
-
- Andrews S. (2010). FastQC A Quality Control tool for High Throughput Sequence Data. URL: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

