Blockade of LAG-3 in PD-L1-Deficient Mice Enhances Clearance of Blood Stage Malaria Independent of Humoral Responses
- PMID: 33519801
- PMCID: PMC7840658
- DOI: 10.3389/fimmu.2020.576743
Blockade of LAG-3 in PD-L1-Deficient Mice Enhances Clearance of Blood Stage Malaria Independent of Humoral Responses
Abstract
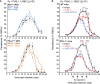
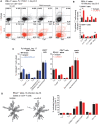
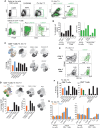
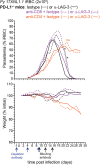
T cells expressing high levels of inhibitory receptors such as PD-1 and LAG-3 are a hallmark of chronic infections and cancer. Checkpoint blockade therapies targeting these receptors have been largely validated as promising strategies to restore exhausted T cell functions and clearance of chronic infections and tumors. The inability to develop long-term natural immunity in malaria-infected patients has been proposed to be at least partially accounted for by sustained expression of high levels of inhibitory receptors on T and B lymphocytes. While blockade or lack of PD-1/PD-L1 and/or LAG-3 was reported to promote better clearance of Plasmodium parasites in various mouse models, how exactly blockade of these pathways contributes to enhanced protection is not known. Herein, using the mouse model of non-lethal P. yoelii (Py) infection, we reveal that the kinetics of blood parasitemia as well as CD4+ T follicular helper (TFH) and germinal center (GC) B cell responses are indistinguishable between PD-1-/-, PD-L1-/- and WT mice. Yet, we also report that monoclonal antibody (mAb) blockade of LAG-3 in PD-L1-/- mice promotes accelerated control of blood parasite growth and clearance, consistent with prior therapeutic blockade experiments. However, neither CD4+ TFH and GC B cell responses, nor parasite-specific Ab serum titers and capacity to transfer protection differed. We also found that i) the majority of LAG-3+ cells are T cells, ii) selective depletion of CD4+ but not CD8+ T cells prevents anti-LAG-3-mediated protection, and iii) production of effector cytokines by CD4+ T cells is increased in anti-LAG-3-treated versus control mice. Thus, taken together, these results are consistent with a model in which blockade and/or deficiency of PD-L1 and LAG-3 on parasite-specific CD4+ T cells unleashes their ability to effectively clear blood parasites, independently from humoral responses.
Keywords: LAG-3+ CD4+ and CD8+ T cells; PD-1/PD-L1/LAG-3; PD-L1 and PD-1 knockout mice; Plasmodium yoelii; checkpoint therapeutic blockade; humoral immunity; inhibitory receptors; malaria.
Copyright © 2021 Furtado, Chorro, Zimmerman, Guillen, Spaulding, Chin, Daily and Lauvau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

