Long-Term Survival of Patients with Metastatic Non-Small-Cell Lung Cancer over Five Decades
- PMID: 33519934
- PMCID: PMC7817269
- DOI: 10.1155/2021/7836264
Long-Term Survival of Patients with Metastatic Non-Small-Cell Lung Cancer over Five Decades
Abstract
Objective: Novel therapeutics and supportive care improved outcomes for metastatic non-small-cell lung cancer (mNSCLC) patients. Major advances over the past five decades include the introduction of combination chemotherapy, small molecules targeting mutant proteins, especially EGFR, and more recently immunotherapy. We aim to document real-world long-term survival over the past five decades.
Methods: Survival statistics were extracted from the Survival, Epidemiology, and End Results (SEER) database for mNSCLC patients during 1973-2015. Two- and five-year survival (2yS and 5yS) were analyzed using Kaplan-Meier and proportional hazard models.
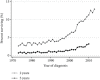
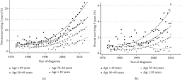
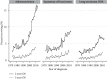
Results: The study population consisted of 280,655mNSCLC patients diagnosed during 1973-2015. Longer survival was seen in younger, female, married, Asian/Pacific Islander race, adenocarcinoma, lower grade, more recent diagnosis, higher income, and chemotherapy-treated patients. 2yS increased during the study period from 2.6% to 12.9%, and 5yS increased from 0.7% to 3.2%. 2yS of patients <50 years of age rose from 2.1% to 22.8%, and their 5yS rose from 0.7% to 6.2%. 2yS of adenocarcinoma patients improved from 2.7% to 16.2%, and their improved 5yS from 1.1% to 3.9%.
Conclusions: Between 1973 and 2015, there was a dramatic improvement in long-term survival, with an approximately five-fold increase in both 2yS and 5yS. Nonetheless, absolute numbers of long-term survivors remained low, with less than 4% living 5 years. This provides a baseline to compare long-term outcomes seen in the current generation of clinical trials.
Copyright © 2021 Jair Bar et al.
Conflict of interest statement
JB reports grants and personal fees from Merck Sharp and Dohme (MSD), Abbvie, AstraZeneca, Pfizer, Takeda, and Roche, grants from Bristol Myers Squibb (BMS), and personal fees from Boehringer Ingelheim (BI), VBL, Novartis, Bayer, and Lilly. AO is a consultant and member of the advisory board to Astra Zeneca, MSD, Roche, and Boehringer Ingelheim. DU reports personal fees from Merck Sharp and Dohme, Bristol Myers Squibb, Boehringer Ingelheim, Roche, Takeda, and AstraZeneca. YL reports research funding from Karyopharm Therapeutics, Checkmate Pharmaceuticals, Bristol-Myers Squibb, and pending from Merck Serono and honoria/consultancy fees from Bristol-Myers Squibb, Clinigen Group and Roche Genetech.
Figures

References
-
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

