Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections
- PMID: 33519949
- PMCID: PMC7834523
- DOI: 10.1016/j.nantod.2020.101051
Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections
Abstract
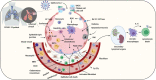
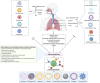
COVID-19, coronavirus disease 2019, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a pandemic. At the time of writing this (October 14, 2020), more than 38.4 million people have become affected, and 1.0 million people have died across the world. The death rate is undoubtedly correlated with the cytokine storm and other pathological pulmonary characteristics, as a result of which the lungs cannot provide sufficient oxygen to the body's vital organs. While diversified drugs have been tested as a first line therapy, the complexity of fatal cases has not been reduced so far, and the world is looking for a treatment to combat the virus. However, to date, and despite such promise, we have received very limited information about the potential of nanomedicine to fight against COVID-19 or as an adjunct therapy in the treatment regimen. Over the past two decades, various therapeutic strategies, including direct-acting antiviral drugs, immunomodulators, a few non-specific drugs (simple to complex), have been explored to treat Acute Respiratory Distress Syndrome (ARDS), Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), influenza, and sometimes the common flu, thus, correlating and developing specific drugs centric to COVID-19 is possible. This review article focuses on the pulmonary pathology caused by SARS-CoV-2 and other viral pathogens, highlighting possible nanomedicine therapeutic strategies that should be further tested immediately.
Keywords: COVID-19; Coronavirus; Influenza, Pulmonary drug delivery; MERS, Nanomedicine; Nanotechnology; Nanotherapeutics; Pathophysiology; SARS, SARS-CoV-2.
© 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. - DOI - PMC - PubMed
-
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
