MELK Inhibition Effectively Suppresses Growth of Glioblastoma and Cancer Stem-Like Cells by Blocking AKT and FOXM1 Pathways
- PMID: 33520717
- PMCID: PMC7842085
- DOI: 10.3389/fonc.2020.608082
MELK Inhibition Effectively Suppresses Growth of Glioblastoma and Cancer Stem-Like Cells by Blocking AKT and FOXM1 Pathways
Erratum in
-
Corrigendum: MELK Inhibition Effectively Suppresses Growth of Glioblastoma and Cancer Stem-Like Cells by Blocking AKT and FOXM1 Pathways.Front Oncol. 2022 Apr 22;12:911817. doi: 10.3389/fonc.2022.911817. eCollection 2022. Front Oncol. 2022. PMID: 35530328 Free PMC article.
Abstract
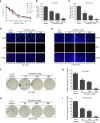
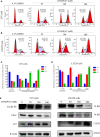
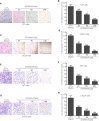
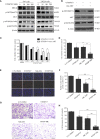
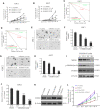
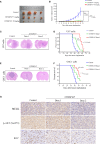
Glioblastoma multiforme (GBM) is a devastating disease yet no effective drug treatment has been established to date. Glioblastoma stem-like cells (GSCs) are insensitive to treatment and may be one of the reasons for the relapse of GBM. Maternal embryonic leucine zipper kinase gene (MELK) plays an important role in the malignant proliferation and the maintenance of GSC stemness properties of GBM. However, the therapeutic effect of targeted inhibition of MELK on GBM remains unclear. This study analyzed the effect of a MELK oral inhibitor, OTSSP167, on GBM proliferation and the maintenance of GSC stemness. OTSSP167 significantly inhibited cell proliferation, colony formation, invasion, and migration of GBM. OTSSP167 treatment reduced the expression of cell cycle G2/M phase-related proteins, Cyclin B1 and Cdc2, while up-regulation the expression of p21 and subsequently induced cell cycle arrest at the G2/M phase. OTSSP167 effectively prolonged the survival of tumor-bearing mice and inhibited tumor cell growth in in vivo mouse models. It also reduced protein kinase B (AKT) phosphorylation levels by OTSSP167 treatment, thereby disrupting the proliferation and invasion of GBM cells. Furthermore, OTSSP167 inhibited the proliferation, neurosphere formation and self-renewal capacity of GSCs by reducing forkhead box M1 (FOXM1) phosphorylation and transcriptional activity. Interestingly, the inhibitory effect of OTSSP167 on the proliferation of GSCs was 4-fold more effective than GBM cells. In conclusion, MELK inhibition suppresses the growth of GBM and GSCs by double-blocking AKT and FOXM1 signals. Targeted inhibition of MELK may thus be potentially used as a novel treatment for GBM.
Keywords: OTSSP167; glioblastoma multiforme; glioblastoma stem-like cells; maternal embryonic leucine-zipper kinase; targeted therapy.
Copyright © 2021 Zhang, Wang, Wang, Liu, Li, Yu, Wu, Liang, Yu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Inhibition of maternal embryonic leucine zipper kinase with OTSSP167 displays potent anti-leukemic effects in chronic lymphocytic leukemia.Oncogene. 2018 Oct;37(41):5520-5533. doi: 10.1038/s41388-018-0333-x. Epub 2018 Jun 12. Oncogene. 2018. PMID: 29895969
-
MELK-dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells.Stem Cells. 2013 Jun;31(6):1051-63. doi: 10.1002/stem.1358. Stem Cells. 2013. PMID: 23404835 Free PMC article.
-
Corrigendum: MELK Inhibition Effectively Suppresses Growth of Glioblastoma and Cancer Stem-Like Cells by Blocking AKT and FOXM1 Pathways.Front Oncol. 2022 Apr 22;12:911817. doi: 10.3389/fonc.2022.911817. eCollection 2022. Front Oncol. 2022. PMID: 35530328 Free PMC article.
-
Maternal embryonic leucine zipper kinase: key kinase for stem cell phenotype in glioma and other cancers.Mol Cancer Ther. 2014 Jun;13(6):1393-8. doi: 10.1158/1535-7163.MCT-13-0764. Epub 2014 May 2. Mol Cancer Ther. 2014. PMID: 24795222 Free PMC article. Review.
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene.BMC Cancer. 2022 Jun 11;22(1):642. doi: 10.1186/s12885-022-09682-2. BMC Cancer. 2022. PMID: 35690717 Free PMC article. Review.
Cited by
-
xCT contributes to colorectal cancer tumorigenesis through upregulation of the MELK oncogene and activation of the AKT/mTOR cascade.Cell Death Dis. 2022 Apr 19;13(4):373. doi: 10.1038/s41419-022-04827-4. Cell Death Dis. 2022. PMID: 35440604 Free PMC article.
-
Forkhead box transcription factors (FOXOs and FOXM1) in glioma: from molecular mechanisms to therapeutics.Cancer Cell Int. 2023 Oct 11;23(1):238. doi: 10.1186/s12935-023-03090-7. Cancer Cell Int. 2023. PMID: 37821870 Free PMC article. Review.
-
DDX56 promotes EMT and cancer stemness via MELK-FOXM1 axis in hepatocellular carcinoma.iScience. 2024 Apr 29;27(6):109827. doi: 10.1016/j.isci.2024.109827. eCollection 2024 Jun 21. iScience. 2024. PMID: 38827395 Free PMC article.
-
AI identifies potent inducers of breast cancer stem cell differentiation based on adversarial learning from gene expression data.Brief Bioinform. 2024 Mar 27;25(3):bbae207. doi: 10.1093/bib/bbae207. Brief Bioinform. 2024. PMID: 38701411 Free PMC article.
-
Comprehensive Analyses of MELK-Associated ceRNA Networks Reveal a Potential Biomarker for Predicting Poor Prognosis and Immunotherapy Efficacy in Hepatocellular Carcinoma.Front Cell Dev Biol. 2022 May 27;10:824938. doi: 10.3389/fcell.2022.824938. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693941 Free PMC article.
References
-
- Stefka A, Park J, Matsuo Y, Chung S, Nakamura Y, Jakubowiak A, et al. . Anti-myeloma activity of MELK inhibitor OTS167: effects on drug-resistant myeloma cells and putative myeloma stem cell replenishment of malignant plasma cells. Blood Cancer J (2016) 6:e460–e. doi: 10.1038/bcj.2016.71 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

