Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions
- PMID: 33520958
- PMCID: PMC7843572
- DOI: 10.3389/fbioe.2020.602659
Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions
Abstract
Point-of-care testing (POCT) allows physicians to detect and diagnose diseases at or near the patient site, faster than conventional lab-based testing. The importance of POCT is considerably amplified in the trying times of the COVID-19 pandemic. Numerous point-of-care tests and diagnostic devices are available in the market including, but not limited to, glucose monitoring, pregnancy and infertility testing, infectious disease testing, cholesterol testing and cardiac markers. Integrating microfluidics in POCT allows fluid manipulation and detection in a singular device with minimal sample requirements. This review presents an overview of two technologies - (a.) Lateral Flow Assay (LFA) and (b.) Nucleic Acid Amplification - upon which a large chunk of microfluidic POCT diagnostics is based, some of their applications, and commercially available products. Apart from this, we also delve into other microfluidic-based diagnostics that currently dominate the in-vitro diagnostic (IVD) market, current testing landscape for COVID-19 and prospects of microfluidics in next generation diagnostics.
Keywords: COVID-19 diagnostics; LFA; NAATs; microfluidics; point-of-care diagnostics.
Copyright © 2021 Sachdeva, Davis and Saha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



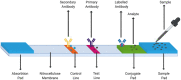
References
-
- Augustine R., Hasan A., Das S., Ahmed R., Mori Y., Notomi T., et al. . (2020). Loop-Mediated Isothermal Amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 9:184. 10.3390/biology9080182 - DOI - PMC - PubMed
-
- Bahadir E. B., Sezgintürk M. K. (2016). Lateral flow assays: principles, designs and labels. TrAC Trends Analy. Chem. 82, 286–306. 10.1016/j.trac.2016.06.006 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

