A Review on Modifications of Amniotic Membrane for Biomedical Applications
- PMID: 33520961
- PMCID: PMC7839407
- DOI: 10.3389/fbioe.2020.606982
A Review on Modifications of Amniotic Membrane for Biomedical Applications
Abstract
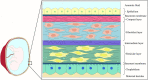
The amniotic membrane (AM) is the innermost layer of the fetal placenta, which surrounds and protects the fetus. Its unique structure, in addition to its physical and biological properties, makes it a useful substance in many applications related to regenerative medicine. The use of this fantastic substance with a century-old history has produced remarkable results in vivo, in vitro, and even in clinical studies. While the intact or preserved AM is widely used for these purposes, the addition of further modifications to AM can be considered as a relatively new subject in its applications. These modifications are applied to improve AM properties, ease of handling, and durability. Here, we will discuss the cases in which AM has undergone additional modifications besides the required processes for sterilization and preservation. In this article, we have categorized these modifications and discussed their applications and results.
Keywords: amniotic membrane; composites; hydrogel; regenerative medicine; tissue engineering.
Copyright © 2021 Dadkhah Tehrani, Firouzeh, Shabani and Shabani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Adamowicz J., Juszczak K., Bajek A., Tworkiewicz J., Nowacki M., Marszalek A., et al. (2012). Morphological and urodynamic evaluation of urinary bladder wall regeneration: muscles guarantee contraction but not proper function—a rat model research study. Transplant. Proc. 44 1429–1434. 10.1016/j.transproceed.2012.01.144 - DOI - PubMed
-
- Alamouti M. A., Shayan N., Momeni M., Koohkan A., Hajizadeh-Saffar E., Nouri M., et al. (2019). Investigation on the safety of amniotic membrane extracts in improving diabetic foot ulcers (phase 1 clinical trial study). Iran. J. Diab. Metabol. 18 0–0.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

