The Impact of miRNAs in Health and Disease of Retinal Pigment Epithelium
- PMID: 33520981
- PMCID: PMC7844312
- DOI: 10.3389/fcell.2020.589985
The Impact of miRNAs in Health and Disease of Retinal Pigment Epithelium
Abstract
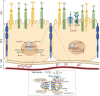
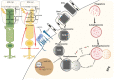
MicroRNAs (miRNAs), a class of non-coding RNAs, are essential key players in the control of biological processes in both physiological and pathological conditions. miRNAs play important roles in fine tuning the expression of many genes, which often have roles in common molecular networks. miRNA dysregulation thus renders cells vulnerable to aberrant fluctuations in genes, resulting in degenerative diseases. The retinal pigment epithelium (RPE) is a monolayer of polarized pigmented epithelial cells that resides between the light-sensitive photoreceptors (PR) and the choriocapillaris. The demanding physiological functions of RPE cells require precise gene regulation for the maintenance of retinal homeostasis under stress conditions and the preservation of vision. Thus far, our understanding of how miRNAs function in the homeostasis and maintenance of the RPE has been poorly addressed, and advancing our knowledge is central to harnessing their potential as therapeutic agents to counteract visual impairment. This review focuses on the emerging roles of miRNAs in the function and health of the RPE and on the future exploration of miRNA-based therapeutic approaches to counteract blinding diseases.
Keywords: AMD; RPE differentiation; miR-204; miR-211; miRNAs; retinal pigment epithelium.
Copyright © 2021 Intartaglia, Giamundo and Conte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adijanto J., Castorino J. J., Wang Z. X., Maminishkis A., Grunwald G. B., Philp N. J. (2012). Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J. Biol. Chem. 287 20491–20503. - PMC - PubMed
-
- Amram B., Cohen-Tayar Y., David A., Ashery-Padan R. (2017). The retinal pigmented epithelium - from basic developmental biology research to translational approaches. Int. J. Dev. Biol. 61 225–234. - PubMed
-
- Anderson D. H., Fisher S. K., Steinberg R. H. (1978). Mammalian cones: disc shedding, phagocytosis, and renewal. Invest. Ophthalmol. Vis. Sci. 17 117–133. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

