Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action
- PMID: 33521324
- PMCID: PMC7829660
- DOI: 10.1038/s41570-020-00244-3
Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action
Abstract
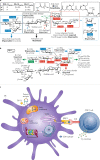
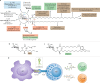
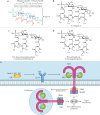
Modern subunit vaccines based on homogeneous antigens offer more precise targeting and improved safety compared with traditional whole-pathogen vaccines. However, they are also less immunogenic and require an adjuvant to increase the immunogenicity of the antigen and potentiate the immune response. Unfortunately, few adjuvants have sufficient potency and low enough toxicity for clinical use, highlighting the urgent need for new, potent and safe adjuvants. Notably, a number of natural and synthetic carbohydrate structures have been used as adjuvants in clinical trials, and two have recently been approved in human vaccines. However, naturally derived carbohydrate adjuvants are heterogeneous, difficult to obtain and, in some cases, unstable. In addition, their molecular mechanisms of action are generally not fully understood, partly owing to the lack of tools to elucidate their immune-potentiating effects, thus hampering the rational development of optimized adjuvants. To address these challenges, modification of the natural product structure using synthetic chemistry emerges as an attractive approach to develop well-defined, improved carbohydrate-containing adjuvants and chemical probes for mechanistic investigation. This Review describes selected examples of natural and synthetic carbohydrate-based adjuvants and their application in synthetic self-adjuvanting vaccines, while also discussing current understanding of their molecular mechanisms of action.
Keywords: Adjuvants; Carbohydrate chemistry; Carbohydrates; Chemical modification; Mechanism of action.
© Springer Nature Limited 2021.
Conflict of interest statement
Competing interestsA.F.-T. is co-inventor on patents and patent applications that include synthetic QS-21 variants mentioned in this work. C.P. and R.F. declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

