Spirocycle MmpL3 Inhibitors with Improved hERG and Cytotoxicity Profiles as Inhibitors of Mycobacterium tuberculosis Growth
- PMID: 33521468
- PMCID: PMC7841955
- DOI: 10.1021/acsomega.0c05589
Spirocycle MmpL3 Inhibitors with Improved hERG and Cytotoxicity Profiles as Inhibitors of Mycobacterium tuberculosis Growth
Abstract
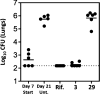
With the emergence of multi-drug-resistant strains of Mycobacterium tuberculosis, there is a pressing need for new oral drugs with novel mechanisms of action. A number of scaffolds with potent anti-tubercular in vitro activity have been identified from phenotypic screening that appear to target MmpL3. However, the scaffolds are typically lipophilic, which facilitates partitioning into hydrophobic membranes, and several contain basic amine groups. Highly lipophilic basic amines are typically cytotoxic against mammalian cell lines and have associated off-target risks, such as inhibition of human ether-à-go-go related gene (hERG) and IKr potassium current modulation. The spirocycle compound 3 was reported to target MmpL3 and displayed promising efficacy in a murine model of acute tuberculosis (TB) infection. However, this highly lipophilic monobasic amine was cytotoxic and inhibited the hERG ion channel. Herein, the related spirocycles (1-2) are described, which were identified following phenotypic screening of the Eli Lilly corporate library against M. tuberculosis. The novel N-alkylated pyrazole portion offered improved physicochemical properties, and optimization led to identification of a zwitterion series, exemplified by lead 29, with decreased HepG2 cytotoxicity as well as limited hERG ion channel inhibition. Strains with mutations in MmpL3 were resistant to 29, and under replicating conditions, 29 demonstrated bactericidal activity against M. tuberculosis. Unfortunately, compound 29 had no efficacy in an acute model of TB infection; this was most likely due to the in vivo exposure remaining above the minimal inhibitory concentration for only a limited time.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- WHO . Global Tuberculosis Report 2020; World Health Organization: Geneva (Switzerland), 2020; ISBN 978-92-4-001313-1.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

