Heat inactivation of the severe acute respiratory syndrome coronavirus 2
- PMID: 33521591
- PMCID: PMC7825878
- DOI: 10.1016/j.jobb.2020.12.001
Heat inactivation of the severe acute respiratory syndrome coronavirus 2
Abstract
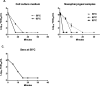
Cell culture medium, nasopharyngeal and sera samples spiked with SARS-CoV-2 were subjected to heat inactivation for various periods of time, ranging from 30 s to 60 min. Our results showed that SARS-CoV-2 could be inactivated in less than 30 min, 15 min, and 3 min at 56 °C, 65 °C, and 95 °C, respectively. These data could help laboratory workers to improve their protocols by handling the virus in biosafety conditions.
Keywords: Covid-19; Heat; Inactivation; SARS-CoV-2.
© 2021 Published by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- WHO-COVID-19-lab_testing-2020.1-eng.pdf. Accessed April 28, 2020. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab...
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
