Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS- CoV-2 vaccine candidates in the Syrian hamster model
- PMID: 33521604
- PMCID: PMC7829205
- DOI: 10.1016/j.isci.2021.102054
Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS- CoV-2 vaccine candidates in the Syrian hamster model
Abstract
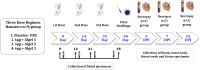
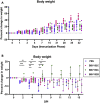
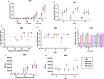
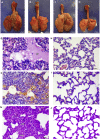
The availability of a safe and effective vaccine would be the eventual measure to deal with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) threat. Here, we have assessed the immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidates BBV152A, BBV152B, and BBV152C in Syrian hamsters. Three dose vaccination regimes with vaccine candidates induced significant titers of SARS-CoV-2-specific IgG and neutralizing antibodies. BBV152A and BBV152B vaccine candidates remarkably generated a quick and robust immune response. Post-SARS-CoV-2 infection, vaccinated hamsters did not show any histopathological changes in the lungs. The protection of the hamster was evident by the rapid clearance of the virus from lower respiratory tract, reduced virus load in upper respiratory tract, absence of lung pathology, and robust humoral immune response. These findings confirm the immunogenic potential of the vaccine candidates and further protection of hamsters challenged with SARS-CoV-2. Of the three candidates, BBV152A showed the better response.
Keywords: Biological Sciences; Immunity; Immunology; Viral Microbiology; Virology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Addetia A., Crawford K.H., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. - DOI - PMC - PubMed
-
- Chan J.F., Zhang A.J., Yuan S., Poon V.K., Chan C.C., Lee A.C., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. Mar. 2020;26:ciaa325. - PMC - PubMed
-
- Ganneru B., Jogdand H., Dharam V.K., Molugu N.R., Prasad S.D., Vellimudu S., Ella K.M., Ravikrishnan R., Awasthi A., Jose J., Rao P. bioRxiv; 2020. Evaluation of Safety and Immunogenicity of an Adjuvanted, TH-1 Skewed, Whole Virion Inactivated SARS-CoV-2 Vaccine-Bbv152. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

