COVID-19 vasculitis and novel vasculitis mimics
- PMID: 33521655
- PMCID: PMC7832717
- DOI: 10.1016/S2665-9913(20)30420-3
COVID-19 vasculitis and novel vasculitis mimics
Abstract
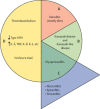
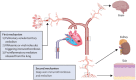
COVID-19 has been occasionally linked to histologically confirmed cutaneous vasculitis and a Kawasaki-like vasculitis, with these entities generally having minimal or no lung involvement and a good prognosis. Unlike these vasculitis types, patients with severe COVID-19 pneumonia can develop cutaneous vasculitis-like lesions and systemic arterial and venous thromboemboli, including cryptogenic strokes and other vasculopathy features. Proposed underlying mechanisms for these severe manifestations have encompassed immune dysregulation, including an anti-phospholipid syndrome-like state, complement activation, viral dissemination with direct systemic endothelial infection, viral RNAaemia with immunothrombosis, clotting pathway activation mediated by hypoxaemia, and immobility. In this Viewpoint, we highlight how imaging and post-mortem findings from patients with COVID-19 indicate a novel thrombosis in the pulmonary venous territory distal to the alveolar capillary bed, a territory that normally acts as a clot filtration system, which might represent an unappreciated nidus for systemic microembolism. Additionally, we suggest that this mechanism represents a novel vasculitis mimic related to COVID-19 that might lead to cryptogenic strokes across multivessel territories, acute kidney injury with haematuria, a skin vasculitis mimic, intestinal ischaemia, and other organ ischaemic manifestations. This finding is supported by pathological reports of extensive pulmonary venular thrombosis and peripheral organ thrombosis with pauci-immune cellular infiltrates. Therefore, severe COVID-19 pneumonia with extensive pulmonary intravascular coagulopathy might help to explain the numerous systemic complications of COVID-19, in which the demonstration of direct organ infection has not adequately explained the pathology.
© 2021 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

