A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice
- PMID: 33521747
- PMCID: PMC7833318
- DOI: 10.1016/j.medj.2020.12.006
A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice
Abstract
Background: The spread of SARS-CoV-2 has caused a worldwide pandemic that has affected almost every aspect of human life. The development of an effective COVID-19 vaccine could limit the morbidity and mortality caused by infection and may enable the relaxation of social-distancing measures. Age is one of the most significant risk factors for poor health outcomes after SARS-CoV-2 infection; therefore, it is desirable that any new vaccine candidates elicit a robust immune response in older adults.
Methods: Here, we use in-depth immunophenotyping to characterize the innate and adaptive immune response induced upon intramuscular administration of the adenoviral vectored ChAdOx1 nCoV-19 (AZD-1222) COVID-19 vaccine candidate in mice.
Findings: A single vaccination generates spike-specific Th1 cells, Th1-like Foxp3+ regulatory T cells, polyfunctional spike-specific CD8+ T cells. and granzyme-B-producing CD8 effectors. Spike-specific IgG and IgM are generated from both the early extrafollicular antibody response and the T follicular helper cell-supported germinal center reaction, which is associated with the production of virus-neutralizing antibodies. A single dose of this vaccine generated a similar type of immune response in aged mice but of a reduced magnitude than in younger mice. We report that a second dose enhances the immune response to this vaccine in aged mice.
Conclusions: This study shows that ChAdOx1 nCoV-19 induces both cellular and humoral immunity in adult and aged mice and suggests a prime-boost strategy is a rational approach to enhance immunogenicity in older persons.
Funding: This study was supported by BBSRC, Lister institute of Preventative Medicine, EPSRC VaxHub, and Innovate UK.
Keywords: CD8+ T cells; COVID-19; T follicular helper cells; Th1 cells; aging; antibodies; germinal center; immunogenicity; vaccination.
© 2020 The Author(s).
Conflict of interest statement
S.C.G. and T.L. are named on a patent application covering ChAdOx1 nCoV-19. The remaining authors declare no competing interests. The funders played no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Figures

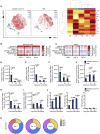
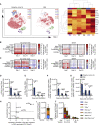
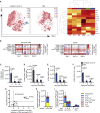


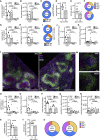
References
-
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00007034/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007038/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_PC_19055/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0428/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

