MicroRNA regulation of cholesterol metabolism
- PMID: 33521946
- PMCID: PMC8938903
- DOI: 10.1111/nyas.14566
MicroRNA regulation of cholesterol metabolism
Abstract
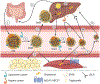
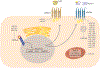
MicroRNAs are small noncoding RNAs that regulate gene expression at the posttranscriptional level. Since many microRNAs have multiple mRNA targets, they are uniquely positioned to regulate the expression of several molecules and pathways simultaneously. For example, the multiple stages of cholesterol metabolism are heavily influenced by microRNA activity. Understanding the scope of microRNAs that control this pathway is highly relevant to diseases of perturbed cholesterol metabolism, most notably cardiovascular disease (CVD). Atherosclerosis is a common cause of CVD that involves inflammation and the accumulation of cholesterol-laden cells in the arterial wall. However, several different cell types participate in atherosclerosis, and perturbations in cholesterol homeostasis may have unique effects on the specialized functions of these various cell types. Therefore, our review discusses the current knowledge of microRNA-mediated control of cholesterol homeostasis, followed by speculation as to how these microRNA-mRNA target interactions might have distinctive effects on different cell types that participate in atherosclerosis.
Keywords: atherosclerosis; cholesterol; metabolism; microRNAs.
© 2021 New York Academy of Sciences.
Figures


References
-
- Ambros V 2004. The functions of animal microRNAs. Nature 431: 350–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

