Ligustrazine induces viability, suppresses apoptosis and autophagy of retinal ganglion cells with ischemia/reperfusion injury through the PI3K/Akt/mTOR signaling pathway
- PMID: 33522374
- PMCID: PMC8806313
- DOI: 10.1080/21655979.2021.1880060
Ligustrazine induces viability, suppresses apoptosis and autophagy of retinal ganglion cells with ischemia/reperfusion injury through the PI3K/Akt/mTOR signaling pathway
Abstract
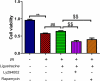
Ligustrazine, an alkaloid monomer extracted from Chuanxiong Rhizoma, has the function of protecting nerve cells. However, the effect and mechanism of ligustrazine on retinal ischemia/reperfusion (I/R) injury still need to be clarified. In our study, retinal ganglion cells (RGC-5) were used to establish a retinal I/R injury model by anaerobic cultivation. Cell viability, autophagy, and apoptosis were evaluated by cell counting kit 8 assay, transmission electron microscopy, and TUNEL staining after treatment with ligustrazine, PI3K inhibitor Ly294002, and/or mTOR inhibitor rapamycin, respectively. Besides, the levels of PI3K/Akt/mTOR pathway and autophagy-related proteins were determined by western blot. Moreover, one-way ANOVA was adopted for inter-group comparisons of measurement data. Our results demonstrated that low-concentration ligustrazine significantly enhanced cell viability and suppressed cell autophagy and apoptosis of RGC-5 cells after I/R injury, suggesting the protective effect of low-concentration ligustrazine on retinal I/R injury. Moreover, the alleviating effect of ligustrazine on RGC-5 with retinal I/R injury was mechanistically associated with the activation of the PI3K/Akt/mTOR pathway. In conclusion, low-concentration ligustrazine has a significant protective effect on RGC-5 cells with retinal I/R injury by activating the PI3K/Akt/mTOR pathway.
Keywords: Ligustrazine; PI3K/Akt/mTOR signaling pathway; autophagy; ischemia/reperfusion injury; retinal ganglion cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
