Evolutionary transcriptomics implicates HAND2 in the origins of implantation and regulation of gestation length
- PMID: 33522483
- PMCID: PMC7943190
- DOI: 10.7554/eLife.61257
Evolutionary transcriptomics implicates HAND2 in the origins of implantation and regulation of gestation length
Abstract
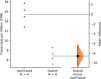
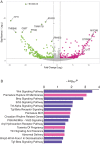
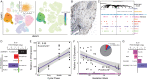
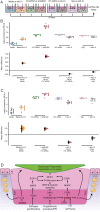
The developmental origins and evolutionary histories of cell types, tissues, and organs contribute to the ways in which their dysfunction produces disease. In mammals, the nature, development and evolution of maternal-fetal interactions likely influence diseases of pregnancy. Here we show genes that evolved expression at the maternal-fetal interface in Eutherian mammals play essential roles in the evolution of pregnancy and are associated with immunological disorders and preterm birth. Among these genes is HAND2, a transcription factor that suppresses estrogen signaling, a Eutherian innovation allowing blastocyst implantation. We found dynamic HAND2 expression in the decidua throughout the menstrual cycle and pregnancy, gradually decreasing to a low at term. HAND2 regulates a distinct set of genes in endometrial stromal fibroblasts including IL15, a cytokine also exhibiting dynamic expression throughout the menstrual cycle and gestation, promoting migration of natural killer cells and extravillous cytotrophoblasts. We demonstrate that HAND2 promoter loops to an enhancer containing SNPs implicated in birth weight and gestation length regulation. Collectively, these data connect HAND2 expression at the maternal-fetal interface with evolution of implantation and gestational regulation, and preterm birth.
Keywords: endometrium; eutheria; evolutionary biology; genetics; genomics; human; marsupial; monotreme; parturition; pregnancy.
© 2021, Marinić et al.
Conflict of interest statement
MM, KM, SC, VL No competing interests declared
Figures





Comment in
-
When the past informs our future.Elife. 2021 Mar 9;10:e67169. doi: 10.7554/eLife.67169. Elife. 2021. PMID: 33686940 Free PMC article.
References
-
- Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, Kiso WK, Schmitt DL, Waddell PJ, Bhaskara S, Jensen ST, Maley CC, Schiffman JD. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. Jama. 2015;314:1850–1860. doi: 10.1001/jama.2015.13134. - DOI - PMC - PubMed
-
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research. 2018;46:W537–W544. doi: 10.1093/nar/gky379. - DOI - PMC - PubMed
-
- Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the 'functional progesterone withdrawal'. Molecular Human Reproduction. 2001;7:581–586. doi: 10.1093/molehr/7.6.581. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

