Mass Spectrometry Quantification, Localization, and Discovery of Feeding-Related Neuropeptides in Cancer borealis
- PMID: 33522802
- PMCID: PMC7906219
- DOI: 10.1021/acschemneuro.1c00007
Mass Spectrometry Quantification, Localization, and Discovery of Feeding-Related Neuropeptides in Cancer borealis
Abstract
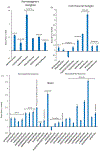
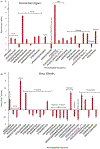
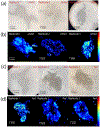
The crab Cancer borealis nervous system is an important model for understanding neural circuit dynamics and modulation, but the identity of neuromodulatory substances and their influence on circuit dynamics in this system remains incomplete, particularly with respect to behavioral state-dependent modulation. Therefore, we used a multifaceted mass spectrometry (MS) method to identify neuropeptides that differentiate the unfed and fed states. Duplex stable isotope labeling revealed that the abundance of 80 of 278 identified neuropeptides was distinct in ganglia and/or neurohemal tissue from fed vs unfed animals. MS imaging revealed that an additional 7 and 11 neuropeptides exhibited altered spatial distributions in the brain and the neuroendocrine pericardial organs (POs), respectively, during these two feeding states. Furthermore, de novo sequencing yielded 69 newly identified putative neuropeptides that may influence feeding state-related neuromodulation. Two of these latter neuropeptides were determined to be upregulated in PO tissue from fed crabs, and one of these two peptides influenced heartbeat in ex vivo preparations. Overall, the results presented here identify a cohort of neuropeptides that are poised to influence feeding-related behaviors, providing valuable opportunities for future functional studies.
Keywords: Mass spectrometry; cardiac neuromodulation; imaging; neuropeptides; stable isotope labeling; stomatogastric nervous system.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures




References
-
- Hulse BK, Jayaraman V Mechanisms underlying the neural computation of head direction. Annu Rev Neurosci 2019, 43, 31–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

