Macrophage Responses to Environmental Stimuli During Homeostasis and Disease
- PMID: 33523133
- PMCID: PMC8284619
- DOI: 10.1210/endrev/bnab004
Macrophage Responses to Environmental Stimuli During Homeostasis and Disease
Abstract
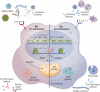
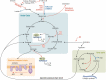
Work over the last 40 years has described macrophages as a heterogeneous population that serve as the frontline surveyors of tissue immunity. As a class, macrophages are found in almost every tissue in the body and as distinct populations within discrete microenvironments in any given tissue. During homeostasis, macrophages protect these tissues by clearing invading foreign bodies and/or mounting immune responses. In addition to varying identities regulated by transcriptional programs shaped by their respective environments, macrophage metabolism serves as an additional regulator to temper responses to extracellular stimuli. The area of research known as "immunometabolism" has been established within the last decade, owing to an increase in studies focusing on the crosstalk between altered metabolism and the regulation of cellular immune processes. From this research, macrophages have emerged as a prime focus of immunometabolic studies, although macrophage metabolism and their immune responses have been studied for centuries. During disease, the metabolic profile of the tissue and/or systemic regulators, such as endocrine factors, become increasingly dysregulated. Owing to these changes, macrophage responses can become skewed to promote further pathophysiologic changes. For instance, during diabetes, obesity, and atherosclerosis, macrophages favor a proinflammatory phenotype; whereas in the tumor microenvironment, macrophages elicit an anti-inflammatory response to enhance tumor growth. Herein we have described how macrophages respond to extracellular cues including inflammatory stimuli, nutrient availability, and endocrine factors that occur during and further promote disease progression.
Keywords: Krebs cycle; epigenetic modifications; immunometabolism; inflammation; macrophage.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643-675. - PubMed
-
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197-216. - PubMed
-
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901-944. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

