Effectiveness of a Female Community Health Volunteer-Delivered Intervention in Reducing Blood Glucose Among Adults With Type 2 Diabetes: An Open-Label, Cluster Randomized Clinical Trial
- PMID: 33523189
- PMCID: PMC7851734
- DOI: 10.1001/jamanetworkopen.2020.35799
Effectiveness of a Female Community Health Volunteer-Delivered Intervention in Reducing Blood Glucose Among Adults With Type 2 Diabetes: An Open-Label, Cluster Randomized Clinical Trial
Abstract
Importance: Female community health volunteers (FCHVs) are frontline community health workers who have been a valuable resource in improving public health outcomes in Nepal, but their value is understudied in diabetes care.
Objective: To assess whether an FCHV-delivered intervention is associated with reduced blood glucose levels among adults with type 2 diabetes.
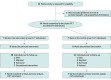
Design, setting, and participants: This community-based, open-label, 2-group, cluster randomized clinical trial with a 12-month delayed control group design was conducted in 14 clusters of a semiurban setting in Western Nepal. A total of 244 adults with type 2 diabetes were recruited between November 2016 and April 2017. The follow-up assessment was conducted at 12 months after enrollment. Data analysis was performed from January to February 2019.
Interventions: Seven clusters were randomized to the FCHV-delivered intervention in which 20 FCHVs provided home visits 3 times a year (once every 4 months) for health promotion counseling and blood glucose monitoring. If participants had blood glucose levels of 126 mg/dL or higher, the FCHVs referred them to the nearest health facility, and if participants were taking antihyperglycemic medication, they were followed up by the FCHVs for adherence to their medication. Seven clusters were randomized to usual care (control group).
Main outcomes and measures: The primary outcome was the change in mean fasting blood glucose from baseline to 12-month follow-up. Secondary outcomes included changes in mean systolic blood pressure, mean diastolic blood pressure, mean body mass index, percentage change in the proportion of low physical activity, harmful alcohol consumption, current smoking, low fruit and vegetable intake, and antihyperglycemic medication status.
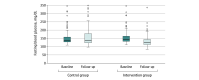
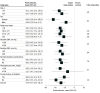
Results: Of 244 participants, 120 women (56.6%) and 92 men (43.4%) completed the trial. At baseline, the mean (SD) age was 51.71 (8.77) years; 127 participants were in the intervention group, and 117 participants were in the control group (usual care). At baseline, the mean (SD) fasting blood glucose level was 156.06 (44.48) mg/dL (158.48 [45.50] mg/dL in the intervention group and 153.43 [43.39] mg/dL in the control group). At 12-month follow-up, the mean fasting blood glucose decreased by 22.86 mg/dL in the intervention group, whereas it increased by 7.38 mg/dL in the control group. The mean reduction was 27.90 mg/dL greater with the intervention (95% CI, -37.62 to -18.18 mg/dL; P < .001). In secondary outcome analyses, there was a greater decline in mean systolic blood pressure in the intervention group than in the control group (-5.40 mm Hg; 95% CI, -8.88 to -1.92 mm Hg; P = .002). There was detectable difference in the intake of antihyperglycemic medication between the groups (relative risk, 1.35; 95% CI, 1.1 to 1.74; P = .02).
Conclusions and relevance: These findings suggest that an FCHV-delivered intervention is associated with reduced blood glucose levels among adults with type 2 diabetes in a low-resource setting in Nepal.
Trial registration: ClinicalTrials.gov Identifier: NCT03304158.
Conflict of interest statement
Figures
References
-
- International Diabetes Federation Diabetes Atlas. 9th ed International Diabetes Federation; 2019.
-
- Aryal KK, Neupane S, Mehata S, et al. . Noncommunicable Diseases Risk Factors: STEPS Survey Nepal 2013. Nepal Health Research Council; 2014.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

