Substrate reduction therapy for inborn errors of metabolism
- PMID: 33523197
- PMCID: PMC7289018
- DOI: 10.1042/ETLS20180058
Substrate reduction therapy for inborn errors of metabolism
Abstract
Inborn errors of metabolism (IEM) represent a growing group of monogenic disorders each associated with inherited defects in a metabolic enzyme or regulatory protein, leading to biochemical abnormalities arising from a metabolic block. Despite the well-established genetic linkage, pathophysiology and clinical manifestations for many IEMs, there remains a lack of transformative therapy. The available treatment and management options for a few IEMs are often ineffective or expensive, incurring a significant burden to individual, family, and society. The lack of IEM therapies, in large part, relates to the conceptual challenge that IEMs are loss-of-function defects arising from the defective enzyme, rendering pharmacologic rescue difficult. An emerging approach that holds promise and is the subject of a flurry of pre-/clinical applications, is substrate reduction therapy (SRT). SRT addresses a common IEM phenotype associated with toxic accumulation of substrate from the defective enzyme, by inhibiting the formation of the substrate instead of directly repairing the defective enzyme. This minireview will summarize recent highlights towards the development of emerging SRT, with focussed attention towards repurposing of currently approved drugs, approaches to validate novel targets and screen for hit molecules, as well as emerging advances in gene silencing as a therapeutic modality.
Keywords: drug discovery and design; gene silencing; inborn errors of metabolism; small molecules; substrate reduction.
© 2019 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures
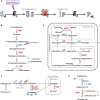
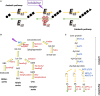
References
LinkOut - more resources
Full Text Sources
