Relative Contributions of All-Trans and 11-Cis Retinal to Formation of Lipofuscin and A2E Accumulating in Mouse Retinal Pigment Epithelium
- PMID: 33523199
- PMCID: PMC7862733
- DOI: 10.1167/iovs.62.2.1
Relative Contributions of All-Trans and 11-Cis Retinal to Formation of Lipofuscin and A2E Accumulating in Mouse Retinal Pigment Epithelium
Abstract
Purpose: Bis-retinoids are a major component of lipofuscin that accumulates in the retinal pigment epithelium (RPE) in aging and age-related macular degeneration (AMD). Although bis-retinoids are known to originate from retinaldehydes required for the light response of photoreceptor cells, the relative contributions of the chromophore, 11-cis retinal, and photoisomerization product, all-trans retinal, are unknown. In photoreceptor outer segments, all-trans retinal, but not 11-cis retinal, is reduced by retinol dehydrogenase 8 (RDH8). Using Rdh8-/- mice, we evaluated the contribution of increased all-trans retinal to the formation and stability of RPE lipofuscin.
Methods: Rdh8-/- mice were reared in cyclic-light or darkness for up to 6 months, with selected light-reared cohorts switched to dark-rearing for the final 1 to 8 weeks. The bis-retinoid A2E was measured from chloroform-methanol extracts of RPE-choroid using HPLC-UV/VIS spectroscopy. Lipofuscin fluorescence was measured from whole flattened eyecups (excitation, 488 nm; emission, 565-725 nm).
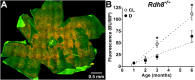
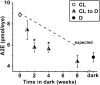
Results: Cyclic-light-reared Rdh8-/- mice accumulated A2E and RPE lipofuscin approximately 1.5 times and approximately 2 times faster, respectively, than dark-reared mice. Moving Rdh8-/- mice from cyclic-light to darkness resulted in A2E levels less than expected to have accumulated before the move.
Conclusions: Our findings establish that elevated levels of all-trans retinal present in cyclic-light-reared Rdh8-/- mice, which remain low in wild-type mice, contribute only modestly to RPE lipofuscin formation and accumulation. Furthermore, decreases in A2E levels occurring after moving cyclic-light-reared Rdh8-/- mice to darkness are consistent with processing of A2E within the RPE and the existence of a mechanism that could be a therapeutic target for controlling A2E cytotoxicity.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal.J Biol Chem. 2012 Jun 22;287(26):22276-86. doi: 10.1074/jbc.M111.329235. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570475 Free PMC article.
-
Exposure of A2E to blue light promotes ferroptosis in the retinal pigment epithelium.Cell Mol Biol Lett. 2025 Feb 21;30(1):22. doi: 10.1186/s11658-025-00700-2. Cell Mol Biol Lett. 2025. PMID: 39984833 Free PMC article.
-
Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina.Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5435-43. doi: 10.1167/iovs.09-3944. Epub 2009 Jun 24. Invest Ophthalmol Vis Sci. 2009. PMID: 19553623 Free PMC article.
-
The 11-cis Retinal Origins of Lipofuscin in the Retina.Prog Mol Biol Transl Sci. 2015;134:e1-12. doi: 10.1016/bs.pmbts.2015.07.022. Prog Mol Biol Transl Sci. 2015. PMID: 26310175 Review.
-
A2E, a byproduct of the visual cycle.Vision Res. 2003 Dec;43(28):2983-90. doi: 10.1016/s0042-6989(03)00475-9. Vision Res. 2003. PMID: 14611934 Review.
Cited by
-
Molecular Genetic Analysis of the Autosomal Recessive Non-Syndromic Inherited Retinitis Pigmentosa.Cureus. 2023 Apr 21;15(4):e37933. doi: 10.7759/cureus.37933. eCollection 2023 Apr. Cureus. 2023. PMID: 37267051 Free PMC article.
-
Bisretinoid lipofuscin, fundus autofluorescence and retinal disease.Prog Retin Eye Res. 2025 Jul 8;108:101388. doi: 10.1016/j.preteyeres.2025.101388. Online ahead of print. Prog Retin Eye Res. 2025. PMID: 40639497 Review.
-
Advances and therapeutic opportunities in visual cycle modulation.Prog Retin Eye Res. 2025 May;106:101360. doi: 10.1016/j.preteyeres.2025.101360. Epub 2025 Apr 23. Prog Retin Eye Res. 2025. PMID: 40280538 Free PMC article. Review.
-
Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina.Antioxidants (Basel). 2023 Dec 13;12(12):2111. doi: 10.3390/antiox12122111. Antioxidants (Basel). 2023. PMID: 38136230 Free PMC article. Review.
-
A Splicing Variant in RDH8 Is Associated with Autosomal Recessive Stargardt Macular Dystrophy.Genes (Basel). 2023 Aug 21;14(8):1659. doi: 10.3390/genes14081659. Genes (Basel). 2023. PMID: 37628710 Free PMC article.
References
-
- Katz ML, Robison WG Jr. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr. 2002; 34: 169–184. - PubMed
-
- Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004; 36: 1400–1404. - PubMed
-
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978; 17: 583–600. - PubMed
-
- Katz ML, Drea CM, Eldred GE, Hess HH, Robison WG Jr. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res. 1986; 43: 561–573. - PubMed
-
- Katz ML, Eldred GE.. Retinal light damage reduces autofluorescent pigment deposition in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1989; 30: 37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

