Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins
- PMID: 33523413
- PMCID: PMC7848667
- DOI: 10.1007/s40259-020-00465-4
Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins
Abstract
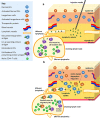
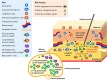
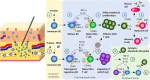
The subcutaneous route of administration has provided convenient and non-inferior delivery of therapeutic proteins compared to intravenous infusion, but there is potential for enhanced immunogenicity toward subcutaneously administered proteins in a subset of patients. Unwanted anti-drug antibody response toward proteins or monoclonal antibodies upon repeated administration is shown to impact the pharmacokinetics and efficacy of multiple biologics. Unique immunogenicity challenges of the subcutaneous route have been realized through various preclinical and clinical examples, although subcutaneous delivery has often demonstrated comparable immunogenicity to intravenous administration. Beyond route of administration as a treatment-related factor of immunogenicity, certain product-related risk factors are particularly relevant to subcutaneously administered proteins. This review attempts to provide an overview of the mechanism of immune response toward proteins administered subcutaneously (subcutaneous proteins) and comments on product-related risk factors related to protein structure and stability, dosage form, and aggregation. A two-wave mechanism of antigen presentation in the immune response toward subcutaneous proteins is described, and interaction with dynamic antigen-presenting cells possessing high antigen processing efficiency and migratory activity may drive immunogenicity. Mitigation strategies for immunogenicity are discussed, including those in general use clinically and those currently in development. Mechanistic insights along with consideration of risk factors involved inspire theoretical strategies to provide antigen-specific, long-lasting effects for maintaining the safety and efficacy of therapeutic proteins.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- U.S. Food and Drug Administration. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products. Silver Spring, MD: FDA; 2014. https://www.fda.gov/downloads/drugs/guidances/ucm338856.pdf Accessed 12 Jun 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

