Confinement-induced self-organization in growing bacterial colonies
- PMID: 33523940
- PMCID: PMC10670964
- DOI: 10.1126/sciadv.abc8685
Confinement-induced self-organization in growing bacterial colonies
Abstract
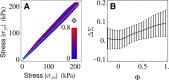
We investigate the emergence of global alignment in colonies of dividing rod-shaped cells under confinement. Using molecular dynamics simulations and continuous modeling, we demonstrate that geometrical anisotropies in the confining environment give rise to an imbalance in the normal stresses, which, in turn, drives a collective rearrangement of the cells. This behavior crucially relies on the colony's solid-like mechanical response at short time scales and can be recovered within the framework of active hydrodynamics upon modeling bacterial colonies as growing viscoelastic gels characterized by Maxwell-like stress relaxation.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Friedl P., Hegerfeldt Y., Tusch M., Collective cell migration in morphogenesis and cancer. Int. J. Dev. Biol. 48, 441–449 (2004). - PubMed
-
- Haeger A., Wolf K., Zegers M. M., Friedl P., Collective cell migration: Guidance principles and hierarchies. Trends Cell Biol. 25, 556–566 (2015). - PubMed
-
- Ben-Jacob E., Cohen I., Gutnick D. L., Cooperative organization of bacterial colonies: From genotype to morphotype. Annu. Rev. Microbiol. 52, 779–806 (1998). - PubMed
-
- Ben-Jacob E., Cohen I., Levine H., Cooperative self-organization of microorganisms. Adv. Phys. 49, 395–554 (2000).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

