A microneedle platform for buccal macromolecule delivery
- PMID: 33523951
- PMCID: PMC10964974
- DOI: 10.1126/sciadv.abe2620
A microneedle platform for buccal macromolecule delivery
Abstract
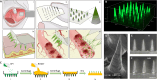
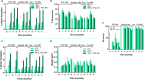
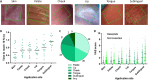
Alternative means for drug delivery are needed to facilitate drug adherence and administration. Microneedles (MNs) have been previously investigated transdermally for drug delivery. To date, drug loading into MNs has been limited by drug solubility in the polymeric blend. We designed a highly drug-loaded MN patch to deliver macromolecules and applied it to the buccal area, which allows for faster delivery than the skin. We successfully delivered 1-mg payloads of human insulin and human growth hormone to the buccal cavity of swine within 30 s. In addition, we conducted a trial in 100 healthy volunteers to assess potential discomfort associated with MNs when applied in the oral cavity, identifying the hard palate as the preferred application site. We envisage that MN patches applied on buccal surfaces could increase medication adherence and facilitate the painless delivery of biologics and other drugs to many, especially for the pediatric and elderly populations.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Fassler D., The fear of needles in children. Am. J. Orthopsychiatry 55, 371–377 (1985). - PubMed
-
- American Academy of Pediatrics Committee on Drugs and Committee on Bioethics , Considerations related to the use of recombinant human growth hormone in children. Pediatrics 99, 122–129 (1997). - PubMed
-
- Committee on Drugs and Committee on Bioethics, AMERICAN ACADEMY OF PEDIATRICS Committee on Drugs and Committee on Bioethics Considerations Related to the Use of Recombinant Human Growth Hormone in Children (1997); www.aappublications.org/news. - PubMed
-
- Harris M., Hofman P. L., Cutfield W. S., Growth hormone treatment in children: Review of safety and efficacy. Paediatr. Drugs 6, 93–106 (2004). - PubMed
-
- Borner M. M., Schoffski P., de Wit R., Caponigro F., Comella G., Sulkes A., Greim G., Peters G. J., van der Born K., Wanders J., de Boer R. F., Martin C., Fumoleau P., Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. Eur. J. Cancer 38, 349–358 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

