Functional Doxorubicin-Loaded Omega-3 Unsaturated Fatty Acids Nanoparticles in Reversing Hepatocellular Carcinoma Multidrug Resistance
- PMID: 33524008
- PMCID: PMC7863563
- DOI: 10.12659/MSM.927727
Functional Doxorubicin-Loaded Omega-3 Unsaturated Fatty Acids Nanoparticles in Reversing Hepatocellular Carcinoma Multidrug Resistance
Abstract
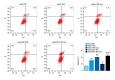
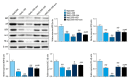
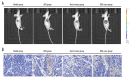
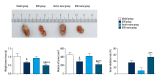
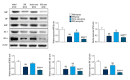
BACKGROUND This study investigated a nanoparticle drug delivery system to reverse multidrug resistance (MDR) and assessed its anticancer efficacy in hepatocellular carcinoma (HCC). MATERIAL AND METHODS Docosahexaenoic acid (DHA) was used as the functional excipient and doxorubicin (DOX) as the chemotherapeutic drug to synthesize DOX nanoparticles (DOX-nano). The human HCC cell line HepG2 was used for experiments. HepG2/DOX, HepG2+DOX, HepG2+DOX-nano, HepG2/DOX+DOX, and HepG2/DOX+DOX-nano groups cells were treated with DOX or DOX-nano (5 μg/mL). Nude mice bearing a HepG2/DOX xenograft were divided into model, DOX, vector-nano, and DOX-nano groups and injected with saline, DOX reagent, vector-nano, and DOX-nano (2 mg/kg), respectively. Next, cytotoxicity, cellular uptake, cell apoptosis and migration, fluorescence imaging, TUNEL assay, and tumor inhibition effects were assessed in vitro and in vivo. Furthermore, expression of MDR-related proteins was also detected using western blotting. RESULTS Fluorescence imaging showed that the DOX uptake in the DOX-nano-treated group was the strongest in the HCC cells or tumors. Cell apoptosis was significantly increased in DOX-nano-treated HepG2/DOX cells and tumors, and cell migration was significantly inhibited in the DOX-nano-treated HepG2/DOX cells compared with the other groups. The tumor inhibitory rate in DOX-nano-injected tumors was also significantly higher than in other groups. The expression of breast cancer resistance protein, B-cell lymphoma 2, lung resistance protein, multidrug resistance protein, and protein kinase C alpha was significantly decreased in DOX-nano-treated HepG2/DOX cells and xenograft tumors. Significantly better antitumor and MDR-reversing effects were also observed in the HepG2+DOX group compared with the HepG2/DOX group. CONCLUSIONS This study revealed the potential efficacy of a DOX-nano drug delivery system for the treatment of HCC, using HepG2/DOX cells and nude mice bearing HepG2/DOX xenografts.
Conflict of interest statement
None.
Figures


Similar articles
-
Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma.Int J Nanomedicine. 2017 Jul 24;12:5271-5287. doi: 10.2147/IJN.S135306. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28769563 Free PMC article.
-
Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance.Mol Pharm. 2014 Jan 6;11(1):59-70. doi: 10.1021/mp400514t. Epub 2013 Nov 21. Mol Pharm. 2014. PMID: 24229050
-
siRNA Targeting of MDR1 Reverses Multidrug Resistance in a Nude Mouse Model of Doxorubicin-resistant Human Hepatocellular Carcinoma.Anticancer Res. 2016 Jun;36(6):2675-82. Anticancer Res. 2016. PMID: 27272776
-
Chemophototherapeutic Ablation of Doxorubicin-Resistant Human Ovarian Tumor Cells.Photochem Photobiol. 2023 Mar;99(2):844-849. doi: 10.1111/php.13677. Epub 2022 Aug 2. Photochem Photobiol. 2023. PMID: 35842741 Free PMC article. Review.
-
Nano-drug delivery system for the treatment of multidrug-resistant breast cancer: Current status and future perspectives.Biomed Pharmacother. 2024 Oct;179:117327. doi: 10.1016/j.biopha.2024.117327. Epub 2024 Aug 30. Biomed Pharmacother. 2024. PMID: 39216449 Review.
Cited by
-
Recent advances in anti-multidrug resistance for nano-drug delivery system.Drug Deliv. 2022 Dec;29(1):1684-1697. doi: 10.1080/10717544.2022.2079771. Drug Deliv. 2022. PMID: 35616278 Free PMC article. Review.
-
Nutraceutical-Based Nanoformulations for Breast and Ovarian Cancer Treatment.Int J Mol Sci. 2022 Oct 10;23(19):12032. doi: 10.3390/ijms231912032. Int J Mol Sci. 2022. PMID: 36233349 Free PMC article. Review.
-
Progress in Research of Nanotherapeutics for Overcoming Multidrug Resistance in Cancer.Int J Mol Sci. 2024 Sep 16;25(18):9973. doi: 10.3390/ijms25189973. Int J Mol Sci. 2024. PMID: 39337463 Free PMC article. Review.
-
Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery.Nutrients. 2022 Oct 12;14(20):4252. doi: 10.3390/nu14204252. Nutrients. 2022. PMID: 36296934 Free PMC article. Review.
-
Human serum albumin-bound paclitaxel nanoparticle inhibits cervical carcinoma cell proliferation and oxidative damage through CYP3A4 and CYP2C8.Heliyon. 2024 Jan 11;10(2):e24460. doi: 10.1016/j.heliyon.2024.e24460. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38347900 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Nooter K, Stoter G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol Res Pract. 1996;192(7):768–80. - PubMed
-
- Chistiakov DA, Myasoedova VA, Orekhov AN. Nanocarriers in improving chemotherapy of multidrug resistant tumors: Key developments and perspectives. Curr Pharm Des. 2017;23(22):3301–8. - PubMed
-
- Lepeltier E, Rijo P, Rizzolio F, et al. Nanomedicine to target multidrug resistant tumors. Drug Resist Updat. 2020;52:100704. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

