Archaeal tyrosine recombinases
- PMID: 33524101
- PMCID: PMC8371274
- DOI: 10.1093/femsre/fuab004
Archaeal tyrosine recombinases
Abstract
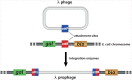
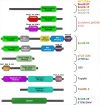
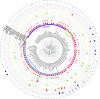
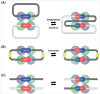
The integration of mobile genetic elements into their host chromosome influences the immediate fate of cellular organisms and gradually shapes their evolution. Site-specific recombinases catalyzing this integration have been extensively characterized both in bacteria and eukarya. More recently, a number of reports provided the in-depth characterization of archaeal tyrosine recombinases and highlighted new particular features not observed in the other two domains. In addition to being active in extreme environments, archaeal integrases catalyze reactions beyond site-specific recombination. Some of these integrases can catalyze low-sequence specificity recombination reactions with the same outcome as homologous recombination events generating deep rearrangements of their host genome. A large proportion of archaeal integrases are termed suicidal due to the presence of a specific recombination target within their own gene. The paradoxical maintenance of integrases that disrupt their gene upon integration implies novel mechanisms for their evolution. In this review, we assess the diversity of the archaeal tyrosine recombinases using a phylogenomic analysis based on an exhaustive similarity network. We outline the biochemical, ecological and evolutionary properties of these enzymes in the context of the families we identified and emphasize similarities and differences between archaeal recombinases and their bacterial and eukaryal counterparts.
Keywords: Archaea; genome evolution; horizontal transfer; mobile genetic element; site-specific recombination; tyrosine recombinase.
© The Author(s) 2021. Published by Oxford University Press on behalf of FEMS.
Figures








References
-
- Abremski K, Gottesman S. Purification of the bacteriophage lambda Xis gene product required for lambda excisive recombination. J Biol Chem. 1982;257:9658–62. - PubMed
-
- Abremski K, Wierzbicki A, Frommer Bet al. Bacteriophage P1 Cre-loxP site-specific recombination. Site-specific DNA topoisomerase activity of the Cre recombination protein. J Biol Chem. 1986;261:391–6. - PubMed
-
- Abremski KE, Hoess RH. Evidence for a second conserved arginine residue in the integrase family of recombination proteins. Protein Eng. 1992;5:87–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

