Structure of a reaction intermediate mimic in t6A biosynthesis bound in the active site of the TsaBD heterodimer from Escherichia coli
- PMID: 33524148
- PMCID: PMC7913687
- DOI: 10.1093/nar/gkab026
Structure of a reaction intermediate mimic in t6A biosynthesis bound in the active site of the TsaBD heterodimer from Escherichia coli
Abstract
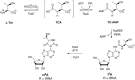
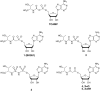
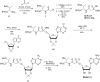
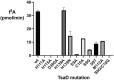
The tRNA modification N6-threonylcarbamoyladenosine (t6A) is universally conserved in all organisms. In bacteria, the biosynthesis of t6A requires four proteins (TsaBCDE) that catalyze the formation of t6A via the unstable intermediate l-threonylcarbamoyl-adenylate (TC-AMP). While the formation and stability of this intermediate has been studied in detail, the mechanism of its transfer to A37 in tRNA is poorly understood. To investigate this step, the structure of the TsaBD heterodimer from Escherichia coli has been solved bound to a stable phosphonate isosteric mimic of TC-AMP. The phosphonate inhibits t6A synthesis in vitro with an IC50 value of 1.3 μM in the presence of millimolar ATP and L-threonine. The inhibitor binds to TsaBD by coordination to the active site Zn atom via an oxygen atom from both the phosphonate and the carboxylate moieties. The bound conformation of the inhibitor suggests that the catalysis exploits a putative oxyanion hole created by a conserved active site loop of TsaD and that the metal essentially serves as a binding scaffold for the intermediate. The phosphonate bound crystal structure should be useful for the rational design of potent, drug-like small molecule inhibitors as mechanistic probes or potentially novel antibiotics.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
Structure-function analysis of an ancient TsaD-TsaC-SUA5-TcdA modular enzyme reveals a prototype of tRNA t6A and ct6A synthetases.Nucleic Acids Res. 2023 Sep 8;51(16):8711-8729. doi: 10.1093/nar/gkad587. Nucleic Acids Res. 2023. PMID: 37427786 Free PMC article.
-
Conformational communication mediates the reset step in t6A biosynthesis.Nucleic Acids Res. 2019 Jul 9;47(12):6551-6567. doi: 10.1093/nar/gkz439. Nucleic Acids Res. 2019. PMID: 31114923 Free PMC article.
-
The structure of the TsaB/TsaD/TsaE complex reveals an unexpected mechanism for the bacterial t6A tRNA-modification.Nucleic Acids Res. 2018 Jun 20;46(11):5850-5860. doi: 10.1093/nar/gky323. Nucleic Acids Res. 2018. PMID: 29741707 Free PMC article.
-
Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t(6)A), a universal modification of tRNA.RNA Biol. 2014;11(12):1529-39. doi: 10.4161/15476286.2014.992277. RNA Biol. 2014. PMID: 25629598 Free PMC article. Review.
-
Conservation and Diversification of tRNA t6A-Modifying Enzymes across the Three Domains of Life.Int J Mol Sci. 2022 Nov 6;23(21):13600. doi: 10.3390/ijms232113600. Int J Mol Sci. 2022. PMID: 36362385 Free PMC article. Review.
Cited by
-
Structure-function analysis of an ancient TsaD-TsaC-SUA5-TcdA modular enzyme reveals a prototype of tRNA t6A and ct6A synthetases.Nucleic Acids Res. 2023 Sep 8;51(16):8711-8729. doi: 10.1093/nar/gkad587. Nucleic Acids Res. 2023. PMID: 37427786 Free PMC article.
-
Structure-function analysis of tRNA t6A-catalysis, assembly, and thermostability of Aquifex aeolicus TsaD2B2 tetramer in complex with TsaE.J Biol Chem. 2024 Dec;300(12):107962. doi: 10.1016/j.jbc.2024.107962. Epub 2024 Nov 5. J Biol Chem. 2024. PMID: 39510188 Free PMC article.
-
The structural and functional workings of KEOPS.Nucleic Acids Res. 2021 Nov 8;49(19):10818-10834. doi: 10.1093/nar/gkab865. Nucleic Acids Res. 2021. PMID: 34614169 Free PMC article. Review.
-
Metal utilization in genome-reduced bacteria: Do human mycoplasmas rely on iron?Comput Struct Biotechnol J. 2021 Oct 18;19:5752-5761. doi: 10.1016/j.csbj.2021.10.022. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34765092 Free PMC article. Review.
-
Commonality and diversity in tRNA substrate recognition in t6A biogenesis by eukaryotic KEOPSs.Nucleic Acids Res. 2022 Feb 28;50(4):2223-2239. doi: 10.1093/nar/gkac056. Nucleic Acids Res. 2022. PMID: 35104889 Free PMC article.
References
-
- Björk G.R. Söll D., RajBhandary U.. Biosynthesis and function of modified nucleosides. tRNA: Structure, Biosynthesis, and Function. 1995; Washington, DC: American Society for Microbiology; 165–205.
-
- El Yacoubi B., Bailly M., de Crecy-Lagard V.. Biosynthesis and function of post-transcriptional modifications of transfer RNAs. Ann. Rev. Gen. 2012; 46:69–95. - PubMed
-
- Schweizer M.P., Chheda G.B., Baczynskyj L., Hall R.H.. Aminoacyl nucleosides. VII. N(purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry. 1969; 8:3283–3289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

