Insight into formation propensity of pseudocircular DNA G-hairpins
- PMID: 33524154
- PMCID: PMC7913771
- DOI: 10.1093/nar/gkab029
Insight into formation propensity of pseudocircular DNA G-hairpins
Abstract
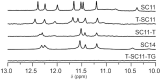
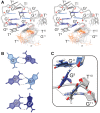
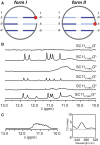
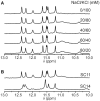
We recently showed that Saccharomyces cerevisiae telomeric DNA can fold into an unprecedented pseudocircular G-hairpin (PGH) structure. However, the formation of PGHs in the context of extended sequences, which is a prerequisite for their function in vivo and their applications in biotechnology, has not been elucidated. Here, we show that despite its 'circular' nature, PGHs tolerate single-stranded (ss) protrusions. High-resolution NMR structure of a novel member of PGH family reveals the atomistic details on a junction between ssDNA and PGH unit. Identification of new sequences capable of folding into one of the two forms of PGH helped in defining minimal sequence requirements for their formation. Our time-resolved NMR data indicate a possibility that PGHs fold via a complex kinetic partitioning mechanism and suggests the existence of K+ ion-dependent PGH folding intermediates. The data not only provide an explanation of cation-type-dependent formation of PGHs, but also explain the unusually large hysteresis between PGH melting and annealing noted in our previous study. Our findings have important implications for DNA biology and nanotechnology. Overrepresentation of sequences able to form PGHs in the evolutionary-conserved regions of the human genome implies their functionally important biological role(s).
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Structural basis for telomeric single-stranded DNA recognition by yeast Cdc13.J Mol Biol. 2004 Apr 23;338(2):241-55. doi: 10.1016/j.jmb.2004.01.063. J Mol Biol. 2004. PMID: 15066429
-
NMR observation of T-tetrads in a parallel stranded DNA quadruplex formed by Saccharomyces cerevisiae telomere repeats.Nucleic Acids Res. 1999 Jun 15;27(12):2457-64. doi: 10.1093/nar/27.12.2457. Nucleic Acids Res. 1999. PMID: 10352174 Free PMC article.
-
Monovalent cation induced structural transitions in telomeric DNAs: G-DNA folding intermediates.Biochemistry. 1991 May 7;30(18):4460-72. doi: 10.1021/bi00232a013. Biochemistry. 1991. PMID: 2021636
-
Interactions of PGH synthase isozymes-1 and -2 with NSAIDs.Ann N Y Acad Sci. 1994 Nov 15;744:50-7. doi: 10.1111/j.1749-6632.1994.tb52723.x. Ann N Y Acad Sci. 1994. PMID: 7825862 Review.
-
Folding of guanine quadruplex molecules-funnel-like mechanism or kinetic partitioning? An overview from MD simulation studies.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt B):1246-1263. doi: 10.1016/j.bbagen.2016.12.008. Epub 2016 Dec 13. Biochim Biophys Acta Gen Subj. 2017. PMID: 27979677 Review.
Cited by
-
Dimeric structures of DNA ATTTC repeats promoted by divalent cations.Nucleic Acids Res. 2024 Feb 28;52(4):1591-1601. doi: 10.1093/nar/gkae052. Nucleic Acids Res. 2024. PMID: 38296828 Free PMC article.
-
Structural Insights into an Antiparallel Chair-Type G-Quadruplex From the Intron of NOP56 Oncogene.Adv Sci (Weinh). 2025 Apr;12(16):e2406230. doi: 10.1002/advs.202406230. Epub 2025 Mar 6. Adv Sci (Weinh). 2025. PMID: 40047221 Free PMC article.
References
-
- Mashimo T., Yagi H., Sannohe Y., Rajendran A., Sugiyama H.. Folding pathways of human telomeric type-1 and type-2 G-Quadruplex structures. J. Am. Chem. Soc. 2010; 132:14910–14918. - PubMed
-
- Zhang M.-L., Xu Y.-P., Kumar A., Zhang Y., Wu W.-Q.. Studying the Potassium-Induced G-Quadruplex DNA folding process using microscale thermophoresis. Biochemistry. 2019; 58:3955–3959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

