Electrostatically Driven Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Material
- PMID: 33524215
- PMCID: PMC10961737
- DOI: 10.1002/anie.202100584
Electrostatically Driven Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Material
Abstract
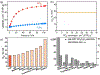
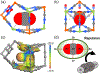
Separating acetylene from carbon dioxide is important but highly challenging owing to their similar physical properties and molecular dimensions. Herein, we report highly efficient electrostatically driven CO2 /C2 H2 separation in an ultramicroporous cadmium nitroprusside (Cd-NP) with compact pore space and complementary electrostatic potential well fitting for CO2 , thus enabling molecular quadrupole moment recognition of CO2 over C2 H2 . This material shows a high CO2 /C2 H2 uptake ratio of 6.0 as well as remarkable CO2 /C2 H2 selectivity of 85 under ambient conditions with modest CO2 heat of adsorption. Neutron powder diffraction experiments and molecular simulations revealed that the electrostatic potential compatibility between pore structure and CO2 allows it to be trapped in a head-on orientation towards the Cd center, whereas the diffusion of C2 H2 is electrostatically forbidden. Dynamic breakthrough experiments have validated the separation performance of this compound for CO2 /C2 H2 separation.
Keywords: cadmium nitroprusside; carbon dioxide; electrostatic potential; gas separation; ultramicroporous materials.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


Similar articles
-
Deciphering Mechanisms of CO2-Selective Recognition over Acetylene within Porous Materials.Chem Bio Eng. 2024 May 13;1(5):366-380. doi: 10.1021/cbe.4c00035. eCollection 2024 Jun 27. Chem Bio Eng. 2024. PMID: 39975798 Free PMC article. Review.
-
Reverse Separation of Carbon Dioxide and Acetylene in Two Isostructural Copper Pyridine-Carboxylate Frameworks.Angew Chem Int Ed Engl. 2024 Jul 22;63(30):e202400823. doi: 10.1002/anie.202400823. Epub 2024 Jun 17. Angew Chem Int Ed Engl. 2024. PMID: 38735839
-
An Ultramicroporous Hydrogen-Bonded Organic Framework Exhibiting High C2 H2 /CO2 Separation.Angew Chem Int Ed Engl. 2022 Oct 24;61(43):e202207579. doi: 10.1002/anie.202207579. Epub 2022 Jul 27. Angew Chem Int Ed Engl. 2022. PMID: 35833470
-
Highly Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Metal-Organic Framework.Adv Mater. 2021 Nov;33(45):e2105880. doi: 10.1002/adma.202105880. Epub 2021 Sep 17. Adv Mater. 2021. PMID: 34535931
-
Ultramicroporous Building Units as a Path to Bi-microporous Metal-Organic Frameworks with High Acetylene Storage and Separation Performance.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13590-13595. doi: 10.1002/anie.201908378. Epub 2019 Aug 13. Angew Chem Int Ed Engl. 2019. PMID: 31407503 Review.
Cited by
-
An adsorbate biased dynamic 3D porous framework for inverse CO2 sieving over C2H2.Chem Sci. 2024 Apr 17;15(20):7698-7706. doi: 10.1039/d3sc06611h. eCollection 2024 May 22. Chem Sci. 2024. PMID: 38784756 Free PMC article.
-
Redox-Responsive Halogen Bonding as a Highly Selective Interaction for Electrochemical Separations.JACS Au. 2024 Jun 10;4(7):2523-2538. doi: 10.1021/jacsau.4c00265. eCollection 2024 Jul 22. JACS Au. 2024. PMID: 39055153 Free PMC article.
-
Deciphering Mechanisms of CO2-Selective Recognition over Acetylene within Porous Materials.Chem Bio Eng. 2024 May 13;1(5):366-380. doi: 10.1021/cbe.4c00035. eCollection 2024 Jun 27. Chem Bio Eng. 2024. PMID: 39975798 Free PMC article. Review.
-
Benchmarking selective capture of trace CO2 from C2H2 using an amine-functionalized adsorbent.Nat Commun. 2025 Mar 16;16(1):2598. doi: 10.1038/s41467-025-57972-7. Nat Commun. 2025. PMID: 40090945 Free PMC article.
-
Old Materials for New Functions: Recent Progress on Metal Cyanide Based Porous Materials.Adv Sci (Weinh). 2022 Jan;9(1):e2104234. doi: 10.1002/advs.202104234. Epub 2021 Nov 26. Adv Sci (Weinh). 2022. PMID: 34825524 Free PMC article. Review.
References
-
- Pässler P, Hefner W, Buckl K, Meinass H, Meiswinkel A, Wernicke HJ, Ebersberg G, Müller R, Bässler J, Behringer H, Mayer D, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000.
-
- Webster CE, Drago RS, Zerner MC, J. Am. Chem. Soc. 1998, 120, 5509–5516.
-
- Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM, Science 2013, 341, 1230444; - PubMed
- Zhao X, Wang Y, Li D-S, Bu X, Feng P, Adv. Mater. 2018, 30, 1705189; - PubMed
- Lin R-B, Xiang S, Zhou W, Chen B, Chem 2020, 6, 337–363;
- Pérez-Botella E, Palomino M, Valencia S, Rey F, Nanoporous Materials for Gas Storage, Springer, Dordrecht, 2019, pp. 173–208;
- Yang S, Ramirez-Cuesta AJ, Newby R, Garcia-Sakai V, Manuel P, Callear SK, Campbell SI, Tang CC, Schröder M, Nat. Chem. 2015, 7, 121–129; - PubMed
- Adil K, Belmabkhout Y, Pillai RS, Cadiau A, Bhatt PM, Assen AH, Maurin G, Eddaoudi M, Chem. Soc. Rev. 2017, 46, 3402–3430; - PubMed
- Hao H-G, Zhao Y-F, Chen D-M, Yu J-M, Tan K, Ma S, Chabal Y, Zhang Z-M, Dou J-M, Xiao Z-H, Day G, Zhou H-C, Lu T-B, Angew. Chem. Int. Ed. 2018, 57, 16067–16071; Angew. Chem. 2018, 130, 16299–16303; - PubMed
- Wang H, Liu Y, Li J, Adv. Mater. 2020, 32, 2002603. - PubMed
-
- Breck DW, Eversole WG, Milton RM, Reed TB, Thomas TL, J. Am. Chem. Soc. 1956, 78, 5972–5977;
- Harper RJ, Stifel GR, Anderson RB, Can. J. Chem. 1969, 47, 4661–4670.
-
- a) Reid CR, Thomas KM, J. Phys. Chem. B 2001, 105, 10619–10629;
- b) Reid CR, Thomas KM, Langmuir 1999, 15, 3206–3218.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

