Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial
- PMID: 33524311
- PMCID: PMC7906655
- DOI: 10.1016/S0140-6736(21)00241-5
Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial
Abstract
Background: As part of the accelerated development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we report a dose-finding and adjuvant justification study of SCB-2019, a protein subunit vaccine candidate containing a stabilised trimeric form of the spike (S)-protein (S-Trimer) combined with two different adjuvants.
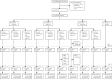
Methods: Our study is a phase 1, randomised, double-blind placebo-controlled trial at a specialised clinical trials centre in Australia. We enrolled healthy adult volunteers in two age groups: younger adults (aged 18-54 years) and older adults (aged 55-75 years). Participants were randomly allocated either vaccine or placebo using a list prepared by the study funder. Participants were to receive two doses of SCB-2019 (either 3 μg, 9 μg, or 30 μg) or a placebo (0·9% NaCl) 21 days apart. SCB-2019 either had no adjuvant (S-Trimer protein alone) or was adjuvanted with AS03 or CpG/Alum. The assigned treatment was administered in opaque syringes to maintain masking of assignments. Reactogenicity was assessed for 7 days after each vaccination. Humoral responses were measured as SCB-2019 binding IgG antibodies and ACE2-competitive blocking IgG antibodies by ELISA and as neutralising antibodies by wild-type SARS-CoV-2 microneutralisation assay. Cellular responses to pooled S-protein peptides were measured by flow-cytometric intracellular cytokine staining. This trial is registered with ClinicalTrials.gov, NCT04405908; this is an interim analysis and the study is continuing.
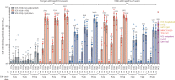
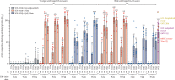
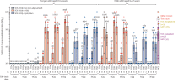
Findings: Between June 19 and Sept 23, 2020, 151 volunteers were enrolled; three people withdrew, two for personal reasons and one with an unrelated serious adverse event (pituitary adenoma). 148 participants had at least 4 weeks of follow-up after dose two and were included in this analysis (database lock, Oct 23, 2020). Vaccination was well tolerated, with two grade 3 solicited adverse events (pain in 9 μg AS03-adjuvanted and 9 μg CpG/Alum-adjuvanted groups). Most local adverse events were mild injection-site pain, and local events were more frequent with SCB-2019 formulations containing AS03 adjuvant (44-69%) than with those containing CpG/Alum adjuvant (6-44%) or no adjuvant (3-13%). Systemic adverse events were more frequent in younger adults (38%) than in older adults (17%) after the first dose but increased to similar levels in both age groups after the second dose (30% in older and 34% in younger adults). SCB-2019 with no adjuvant elicited minimal immune responses (three seroconversions by day 50), but SCB-2019 with fixed doses of either AS03 or CpG/Alum adjuvants induced high titres and seroconversion rates of binding and neutralising antibodies in both younger and older adults (anti-SCB-2019 IgG antibody geometric mean titres at day 36 were 1567-4452 with AS03 and 174-2440 with CpG/Alum). Titres in all AS03 dose groups and the CpG/Alum 30 μg group were higher than were those recorded in a panel of convalescent serum samples from patients with COVID-19. Both adjuvanted SCB-2019 formulations elicited T-helper-1-biased CD4+ T-cell responses.
Interpretation: The SCB-2019 vaccine, comprising S-Trimer protein formulated with either AS03 or CpG/Alum adjuvants, elicited robust humoral and cellular immune responses against SARS-CoV-2, with high viral neutralising activity. Both adjuvanted vaccine formulations were well tolerated and are suitable for further clinical development.
Funding: Clover Biopharmaceuticals and the Coalition for Epidemic Preparedness Innovations.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Next-generation COVID-19 vaccines: here come the proteins.Lancet. 2021 Feb 20;397(10275):643-645. doi: 10.1016/S0140-6736(21)00258-0. Epub 2021 Jan 29. Lancet. 2021. PMID: 33524312 Free PMC article. No abstract available.
References
-
- Johns Hopkins University of Medicine Coronavirus Resource Center COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Jan 23, 2021. https://coronavirus.jhu.edu/map.html
-
- WHO Draft landscape of COVID-19 candidate vaccines. Jan 22, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

