Measuring functional connectivity with wearable MEG
- PMID: 33524584
- PMCID: PMC8216250
- DOI: 10.1016/j.neuroimage.2021.117815
Measuring functional connectivity with wearable MEG
Abstract
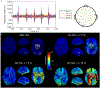
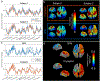
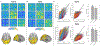
Optically-pumped magnetometers (OPMs) offer the potential for a step change in magnetoencephalography (MEG) enabling wearable systems that provide improved data quality, accommodate any subject group, allow data capture during movement and potentially reduce cost. However, OPM-MEG is a nascent technology and, to realise its potential, it must be shown to facilitate key neuroscientific measurements, such as the characterisation of brain networks. Networks, and the connectivities that underlie them, have become a core area of neuroscientific investigation, and their importance is underscored by many demonstrations of their disruption in brain disorders. Consequently, a demonstration of network measurements using OPM-MEG would be a significant step forward. Here, we aimed to show that a wearable 50-channel OPM-MEG system enables characterisation of the electrophysiological connectome. To this end, we measured connectivity in the resting state and during a visuo-motor task, using both OPM-MEG and a state-of-the-art 275-channel cryogenic MEG device. Our results show that resting-state connectome matrices from OPM and cryogenic systems exhibit a high degree of similarity, with correlation values >70%. In addition, in task data, similar differences in connectivity between individuals (scanned multiple times) were observed in cryogenic and OPM-MEG data, again demonstrating the fidelity of the OPM-MEG device. This is the first demonstration of network connectivity measured using OPM-MEG, and results add weight to the argument that OPMs will ultimately supersede cryogenic sensors for MEG measurement.
Keywords: AEC; Amplitude-envelope correlation; Functional connectivity; MEG; Magnetoencephalography; Network; OPM; OPM-MEG; Optically-pumped magnetometer; Wearable MEG.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest V.S. is the founding director of QuSpin, the commercial entity selling OPM magnetometers. J.O. is an employee of QuSpin. E.B. and M.J.B. are directors of Cerca Magnetics Limited, a newly established spin-out company whose aim is to commercialise aspects of OPM-MEG technology. E.B., M.J.B., R.B., N.H. and R.H. hold founding equity in Cerca Magnetics Limited.
Figures


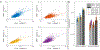

Comment in
-
Whole-head OPM-MEG enables noninvasive assessment of functional connectivity.Trends Neurosci. 2021 Jul;44(7):510-512. doi: 10.1016/j.tins.2021.04.006. Epub 2021 May 5. Trends Neurosci. 2021. PMID: 33965213
References
-
- Altarev I, Fierlinger P, Lins T, Marino MG, Nießen B, Petzoldt G, Reisner M, Stuiber S, Sturm M, Taggart Singh J, Taubenheim B, Rohrer HK, Schläpfer U, 2015. Minimizing magnetic fields for precision experiments. J. Appl. Phys 117. doi:10.1063/1.4922671. - DOI
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B doi:10.1111/j.2517-6161.1995.tb02031.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

