Diabetes and hypertension: Pivotal involvement of purinergic signaling
- PMID: 33524787
- PMCID: PMC7846467
- DOI: 10.1016/j.biopha.2021.111273
Diabetes and hypertension: Pivotal involvement of purinergic signaling
Abstract
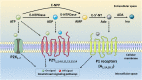
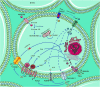
Diabetes mellitus (DM) and hypertension are highly prevalent worldwide health problems and frequently associated with severe clinical complications, such as diabetic cardiomyopathy, nephropathy, retinopathy, neuropathy, stroke, and cardiac arrhythmia, among others. Despite all existing research results and reasonable speculations, knowledge about the role of purinergic system in individuals with DM and hypertension remains restricted. Purinergic signaling accounts for a complex network of receptors and extracellular enzymes responsible for the recognition and degradation of extracellular nucleotides and adenosine. The main components of this system that will be presented in this review are: P1 and P2 receptors and the enzymatic cascade composed by CD39 (NTPDase; with ATP and ADP as a substrate), CD73 (5'-nucleotidase; with AMP as a substrate), and adenosine deaminase (ADA; with adenosine as a substrate). The purinergic system has recently emerged as a central player in several physiopathological conditions, particularly those linked to inflammatory responses such as diabetes and hypertension. Therefore, the present review focuses on changes in both purinergic P1 and P2 receptor expression as well as the activities of CD39, CD73, and ADA in diabetes and hypertension conditions. It can be postulated that the manipulation of the purinergic axis at different levels can prevent or exacerbate the insurgency and evolution of diabetes and hypertension working as a compensatory mechanism.
Keywords: ATP; Adenosine; Blood pressure; Ectonucleotidases; Glucose; Purinergic receptors.
Copyright © 2021 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures

References
-
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. - DOI - PMC - PubMed
-
- Baynest H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015;6(5):1–9. doi: 10.4172/2155-6156.1000541. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

