Epstein-Barr virus latent membrane protein-1 upregulates autophagy and promotes viability in Hodgkin lymphoma: Implications for targeted therapy
- PMID: 33525055
- PMCID: PMC8019199
- DOI: 10.1111/cas.14833
Epstein-Barr virus latent membrane protein-1 upregulates autophagy and promotes viability in Hodgkin lymphoma: Implications for targeted therapy
Abstract
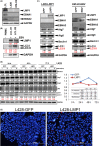
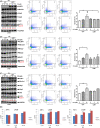
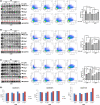
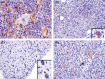
Hodgkin lymphoma (HL) is composed of neoplastic Hodgkin and Reed-Sternberg cells in an inflammatory background. The neoplastic cells are derived from germinal center B cells that, in most cases, are infected by Epstein-Barr virus (EBV), which may play a role in tumorigenesis. Given that EBV-latent membrane protein 1 (LMP1) regulates autophagy in B cells, we explored the role of autophagy mediated by EBV or LMP1 in HL. We found that EBV-LMP1 transfection in HL cells induced a modest increase in autophagy signals, attenuated starvation-induced autophagic stress, and alleviated autophagy inhibition- or doxorubicin-induced cell death. LMP1 knockdown leads to decreased autophagy LC3 signals. A xenograft mouse model further showed that EBV infection significantly increased expression of the autophagy marker LC3 in HL cells. Clinically, LC3 was expressed in 15% (19/127) of HL samples, but was absent in all cases of nodular lymphocyte-predominant and lymphocyte-rich classic HL cases. Although expression of LC3 was not correlated with EBV status or clinical outcome, autophagic blockade effectively eradicated LMP1-positive HL xenografts with better efficacy than LMP1-negative HL xenografts. Collectively, these results suggest that EBV-LMP1 enhances autophagy and promotes the viability of HL cells. Autophagic inhibition may be a potential therapeutic strategy for treating patients with HL, especially EBV-positive cases.
Keywords: Epstein-Barr virus; Hodgkin lymphoma; autophagy; chloroquine; latent membrane protein-1; xenograft.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Diehl V, Thomas RK, Re D. Part II: Hodgkin's lymphoma–diagnosis and treatment. Lancet Oncol. 2004;5(1):19‐26. - PubMed
-
- Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9(1):15‐27. - PubMed
-
- Ushmorov A, Leithauser F, Sakk O, et al. Epigenetic processes play a major role in B‐cell‐specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107(6):2493‐2500. - PubMed
-
- Piris MA, Medeiros LJ, Chang KC. Hodgkin lymphoma: a review of pathological features and recent advances in pathogenesis. Pathology. 2020;52(1):154‐165. - PubMed
-
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739‐789. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

