Pharmacokinetics and pharmacodynamics of therapeutic antibodies in tumors and tumor-draining lymph nodes
- PMID: 33525083
- PMCID: PMC7935407
- DOI: 10.3934/mbe.2021006
Pharmacokinetics and pharmacodynamics of therapeutic antibodies in tumors and tumor-draining lymph nodes
Abstract
The signaling axis from the primary tumor to the tumor-draining lymph node (TDLN) has emerged as a crucial mediator for the efficacy of immunotherapies in neoadjuvant settings, challenging the primary use of immunotherapy in adjuvant settings. TDLNs are regarded as highly opportunistic sites for cancer cell dissemination and promote further spread via several primary tumor-dependent mechanisms. Lesion-level mixed responses to antibody immunotherapy have been traced to local immune signatures present in the TDLN and the organ-specific primary tumors that they drain. However, the pharmacokinetics (PK) and biodistribution gradients of antibodies in primary tumors and TDLNs have not been systemically evaluated. These concentration gradients are critical in ensuring adequate antibody pharmacodynamic (PD) T-cell activation and/or anti-tumor response. The current work reviews the knowledge for developing physiologically-based PK and pharmacodynamic (PBPK/PD) models to quantify antibody biodistribution gradients in anatomically distinct primary tumors and TDLNs as a means to characterize the clinically observed heterogeneous responses to antibody therapies. Several clinical and pathophysiological considerations in modeling the primary tumor-TDLN axis, as well as a summary of both preclinical and clinical PK/PD lymphatic antibody disposition studies, will be provided.
Keywords: PK/PD modeling; monoclonal antibody (mAb); physiologically-based pharmacokinetic (PBPK) models; target-mediated drug disposition (TMDD); tumor-draining lymph nodes (TDLNs).
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest. E.S. is a postdoctoral fellow sponsored by the UNC/IQVIA fellowship.
Figures
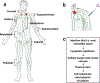
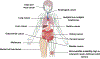


References
-
- Brooks M Cancer Drugs Dominate Top 10 Best-Selling Drugs in 2018. Medscape Medical News: WebMD.(2019), pp.
-
- Press release: The Nobel Prize in Physiology or Medicine 2018, In: NobelPrize.org, editor: NobelPrize.org.(2018), pp.
-
- Dirks NL and Meibohm B, Population Pharmacokinetics of Therapeutic Monoclonal Antibodies, Clinical Pharmacokinetics, 49 (2010), 633–659. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
