Neurometals in the Pathogenesis of Prion Diseases
- PMID: 33525334
- PMCID: PMC7866166
- DOI: 10.3390/ijms22031267
Neurometals in the Pathogenesis of Prion Diseases
Abstract
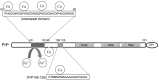
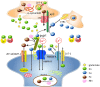
Prion diseases are progressive and transmissive neurodegenerative diseases. The conformational conversion of normal cellular prion protein (PrPC) into abnormal pathogenic prion protein (PrPSc) is critical for its infection and pathogenesis. PrPC possesses the ability to bind to various neurometals, including copper, zinc, iron, and manganese. Moreover, increasing evidence suggests that PrPC plays essential roles in the maintenance of homeostasis of these neurometals in the synapse. In addition, trace metals are critical determinants of the conformational change and toxicity of PrPC. Here, we review our studies and other new findings that inform the current understanding of the links between trace elements and physiological functions of PrPC and the neurotoxicity of PrPSc.
Keywords: Alzheimer’s disease; amyloid; calcium homeostasis; dementia with Lewy bodies; neurotoxicity; synapse.
Conflict of interest statement
All authors declare there are no conflict of interest.
Figures

References
-
- Alafuzoff I., Hartikainen P. Alpha-synucleinopathies. Handb. Clin. Neurol. 2017;145:339–353. - PubMed
-
- Kaźmierczak A., Czapski G.A., Adamczyk A., Gajkowska B., Strosznajder J.B. A novel mechanism of non-Aβ component of Alzheimer’s disease amyloid (NAC) neurotoxicity. Interplay between p53 protein and cyclin-dependent kinase 5 (Cdk5) Neurochem. Int. 2011;58:206–214. doi: 10.1016/j.neuint.2010.11.018. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

