The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer's Disease
- PMID: 33525357
- PMCID: PMC7865740
- DOI: 10.3390/ijms22031273
The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer's Disease
Abstract
Neurodegenerative diseases are a major public health problem worldwide with a wide spectrum of symptoms and physiological effects. It has been long reported that the dysregulation of the cholinergic system and the adrenergic system are linked to the etiology of Alzheimer's disease. Cholinergic neurons are widely distributed in brain regions that play a role in cognitive functions and normal cholinergic signaling related to learning and memory is dependent on acetylcholine. The Locus Coeruleus norepinephrine (LC-NE) is the main noradrenergic nucleus that projects and supplies norepinephrine to different brain regions. Norepinephrine has been shown to be neuroprotective against neurodegeneration and plays a role in behavior and cognition. Cholinergic and adrenergic signaling are dysregulated in Alzheimer's disease. The degeneration of cholinergic neurons in nucleus basalis of Meynert in the basal forebrain and the degeneration of LC-NE neurons were reported in Alzheimer's disease. The aim of this review is to describe current literature on the role of the cholinergic system and the adrenergic system (LC-NE) in the pathology of Alzheimer's disease and potential therapeutic implications.
Keywords: Alzheimer; Locus Coeruleus; acetylcholine; adrenergic; cholinergic; cognition; epigenetics; norepinephrine; signaling.
Conflict of interest statement
The author declares no conflict of interest.
Figures
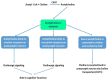
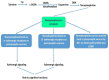

References
-
- Products—Data Briefs—Number 355—January 2020. [(accessed on 7 October 2020)]; Available online: https://www.cdc.gov/nchs/products/databriefs/db355.htm.
-
- Wenk G.L. Neuropathologic Changes in Alzheimer’s Disease. J. Clin. Psychiatry. 2003;64(Suppl. 9):7–10. - PubMed
-
- Hampel H., Mesulam M.-M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain. 2018;141:1917–1933. doi: 10.1093/brain/awy132. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

