The Role of GSH in Intracellular Iron Trafficking
- PMID: 33525417
- PMCID: PMC7865746
- DOI: 10.3390/ijms22031278
The Role of GSH in Intracellular Iron Trafficking
Abstract
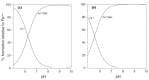
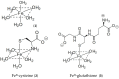
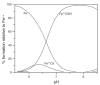
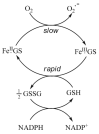
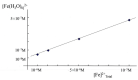
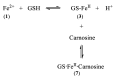
Evidence is reviewed for the role of glutathione in providing a ligand for the cytosolic iron pool. The possibility of histidine and carnosine forming ternary complexes with iron(II)glutathione is discussed and the physiological significance of these interactions considered. The role of carnosine in muscle, brain, and kidney physiology is far from established and evidence is presented that the iron(II)-binding capability of carnosine relates to this role.
Keywords: carnosine; cytosol; glutathione; histidine; labile iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lankford C.D. Bacterial Assimilation of iron. CRC Rev. Microbiol. 1973;2:273–331. doi: 10.3109/10408417309108388. - DOI
-
- Marschner H. Mineral Nutrition of Higher Plants. 2nd ed. Academic Press; London, UK: 1993.
-
- Crichton R. Iron Metabolism. 3rd ed. Wiley; Chichester, UK: 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

