An Injectable Nano-Enabled Thermogel to Attain Controlled Delivery of p11 Peptide for the Potential Treatment of Ocular Angiogenic Disorders of the Posterior Segment
- PMID: 33525495
- PMCID: PMC7910951
- DOI: 10.3390/pharmaceutics13020176
An Injectable Nano-Enabled Thermogel to Attain Controlled Delivery of p11 Peptide for the Potential Treatment of Ocular Angiogenic Disorders of the Posterior Segment
Abstract
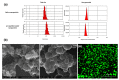
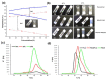
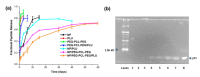
This investigation focused on the design of an injectable nano-enabled thermogel (nano-thermogel) system to attain controlled delivery of p11 anti-angiogenic peptide for proposed effective prevention of neovascularisation and to overcome the drawbacks of the existing treatment approaches for ocular disorders characterised by angiogenesis, which employ multiple intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) antibodies. Synthesis of a polyethylene glycol-polycaprolactone-polyethylene glycol (PEG-PCL-PEG) triblock co-polymer was undertaken, followed by characterisation employing Fourier-transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy and differential scanning calorimetry (DSC) to ascertain the chemical stability and integrity of the co-polymer instituted for nano-thermogel formulation. The p11 anti-angiogenic peptide underwent encapsulation within poly(lactic-co-glycolic acid) (PLGA) nanoparticles via a double emulsion solvent evaporation method and was incorporated into the thermogel following characterisation by scanning electron microscopy (SEM), zeta size and zeta-potential analysis. The tube inversion approach and rheological analysis were employed to ascertain the thermo-sensitive sol-gel conversion of the nano-thermogel system. Chromatographic assessment of the in vitro release of the peptide was performed, with stability confirmation via Tris-Tricine PAGE (Polyacrylamide Gel Electrophoresis). In vitro biocompatibility of the nano-thermogel system was investigated employing a retinal cell line (ARP-19). A nanoparticle size range of 100-200 nm and peptide loading efficiency of 67% was achieved. Sol-gel conversion of the nano-thermogel was observed between 32-45 °C. Release of the peptide in vitro was sustained, with maintenance of stability, for 60 days. Biocompatibility assessment highlighted 97-99% cell viability with non-haemolytic ability, which supports the potential applicability of the nano-thermogel system for extended delivery of peptide for ocular disorder treatment.
Keywords: angiogenesis; nanoparticles; ocular drug delivery; peptide; thermosensitive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

